Review article
Vol.8 Issue.1 Page No. 46-69
Farah Deeba1,2*, Minal Bafna3, Ankur Jain2,4
1S S Jain Subodh P G College, Jaipur, India
2School of Applied Sciences, Suresh GyanVihar University, Jaipur, India
3Department of Physics, Agrawal P. G. College, Jaipur, India
4Center for Renewable Energy and Storage, Suresh GyanVihar University, Jaipur, India
*Corresponding authors email: *mariya2deeba@gmail.com
Received: 29/09/2021 Published – 10/02/2022
Keywords:
Polymer Blends,
Composites,
Dielectric spectroscopy,
optoelectronic devices
Abstract:
During the last few decades, researchers have shown their interest and attention towards the materials which possess enhanced electrical and optical properties with suitable mechanical strength. The research focused on modern technologies on the new materials and blends having distinct ‘amalgamations properties. In this context, different polymers/polymer blends/polymer composites have been explored with their different electrical and optical behaviour for the wide frequency ranges from 20Hz to 1MHz which showed different characteristic and behaviour in different audio frequency (AF) and low radio frequency (RF) ranges. In this article, the efforts on improving the electrical and structural properties of different polymer/polymer blend or composites by filling with salts and inorganic fillers like PVA –ZnO, PVA-TiO2, PMMA-TiO2, PEO- Al2O3, PVA- SiO2, PMMA- Metal Oxides(ZnO, SiO2, CuO, ZnS, TiO2& Al2O3),PVA-C-ZnO, PVA-PEO – ZnO, PPY-PVA- Metal Oxides(ZnO,SnO2& TiO2)PEO-PMMA-Salt-MMT(Mont Morillonite), PEO-PVP- SiO2, PVP- EGO(Ethylene Glycol Oligomer), PEO- OMMT(Organophilic MMT), PEO- MMT, PMMA-PEO- Salt- MMT, PMMA-PEG-MMT, PEO–PMMA-SiO2 are reviewed.
- Introduction:
In the last few years, a special attraction towards the new conducting materials is drawn. These materials have fast ion conducting properties with conductivities range of 10-5 to 10-1 S cm-1 at ambient temperature. Such materials are often termed as ‘Super Ionic Solids’ or ‘Solid Electrolytes’ or ‘Fast Ion Conductors’ depending on their applications. On the basis of their structures, compositions, phases, physical properties, these superionic solids are classified broadly into distinguish classes such as ‘crystalline or polycrystalline’, ‘amorphous or glassy electrolytes’, ‘blend or composite electrolytes’ and polymer electrolytes[1-3]. The recent studies and reviews revealed the use of different polymer electrolytes with or without nanofillers or salts. These polymer electrolytes are attracting great attention for their synthesis, fabrication and applications in various electronic or microelectronics devices, electrochemical devices because of their unique properties like less dielectric losses, high ionic conductivity, tuned mechanical strength and fine flexibility. Some of the metal oxides having a good absorbing quality when doped in polymer are used as absorbing materials for electromagnetic shielding. Stability and thin film casting of these materials has wide applications. The solid polymer electrolytes (SPEs) have lower conductivity because of high restriction in the motion of the ‘polymer molecules’, which effects the efficiency of the performance of such devices made of SPEs. This causes the development of ‘gel’ or ‘plasticized’ polymer electrolytes, whose conductivity is in the similar range as that of the ‘liquid electrolytes’. But these materials again suffer from weak mechanical strength &stability due to the presence of ‘volatile solvents’ in it [3-6]. To reduce such gap, numerous modified chemical and physical techniques have been adopted by the researchers like polymer blending and doping in their researches. Basically, the polymer blending is a mixture of two or more polymers in different ratios, in which, one absorbs the EAS (Electrolyte Active Species) while another remains inert i.e. un-dissolved. The second phase provides toughness to the polymer blend films [7].It has been found that, by combining inorganic and organic components either at the molecular level or in their partially condensed forms, the mechanical, chemical, or physical properties of these composites can be controlled intelligently over wide ranges. These properties are highly influenced by the nature of the constituents as well as the reaction pathway chosen for their preparation. In general, interactions between the inorganic and organic components of the composite can range from weak forces such as hydrogen bonding to strong covalent bonds [8].
2.Polymer Families for Polymer Nanocomposites(PNCs) films
2.1 PolyAniline(PANI):
In the electrically conducting polymer family, polyaniline(PANI) is the most popular conducting polymer due toits properties, such as easy synthesis, low cost, stability, unique doping process and possessing wide range of conductivity upon doping [9-13]. However, the process ability of PANI has limitations i.e. the powdered form of PANI does not dissolve in any ‘common organic solvents’ and the polymer is degraded at high temperature and results in poor mechanical stability [14-16].To sort out the limitations of the process ability of the PANI, various methods have been discussed, blending and forming composites are the two most commonly used techniques [15-18]. In1990, after the discovery of the solution process ability of PANI, research on PANI composites or blends with insulating polymers gained popularity [17-18]. Over the years, many studies have been performed to find the substitute of conventional inorganic conductive fillers, such as metal particles and conducting carbon black, with conducting PANI fillers [19-21]. A number of research groups have performed extensive work on different synthesis methods and properties of various polymer blends and composites with insulting polymer matrices [22].“Thermo set is a stronger polymer compared to ‘thermoplastic’ because it’s cross linking is distributed in all three directions”[23]. Thus, thermo set polymers are suitable for high temperature and toxic chemical environment applications because they can maintain their shape and size due to the strong covalent bonds between the polymer chains and cannot be easily broken [22-24].
2.2 PolyMethyl Methacrylate (PMMA):
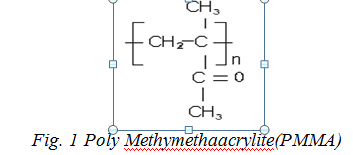
PMMA attracts a great attention because of its fine optical properties and vast use in optical and electronic devices. Different properties of PMMA have been studied after doping with various fillers including malachite green-doped PMMA, which reflects its use in microelectronics. Polymethyl methacrylate (PMMA) was discovered by British chemists Rowland Hill and John Crawford at Imperial Chemical Industries (ICI) in the United Kingdom (Early 1930s), its structure is shown in below Fig. 1 [8, 22-23]. PMMA is a plastic, widely used for its stiffness and clarity in various industrial fields. It can be used as a good keeper for rare earth iron garnets which has wide technological application. The structures of pristine and doped PMMA were investigated using analysis of their infrared spectra and wide-angle XRD analysis [25]. Its pristine form has two broad humps showing its amorphous nature. The structure characteristics of PMMA have been the subject of several investigations when it is blended with some other polymers or doped with inorganic fillers. Its morphological study reflects its structural change in its matrix which do affects its mechanical, optical and electrical properties.
By using PMMA as a host polymer and C6H10O4 as doping agent PMMA/ C6H10O4was prepared by solution casting technique using DMF as solvent. The FTIR data and analysis revealed the complex formation between the salt and polymer matrix. The spectra analysis revealed that with the incorporation of adipic acid (salt) to pristine PMMA, dc conductivity value of pristine PMMA increases at ambient temperature. The ‘Magnitude Bode ploy’ shows the decline in the impedance with the addition of adipic acid to pristine PMMA[26].Also, for PMMA with PVDF, the XRD analysis revealed that the blends take place based on the influence of PMMA content on PVDF blends and varies with their different % of weight ratios. PMMA as a host polymer when blended with other polymers like PVA, PEO, PVDF in different ratios, their enhanced or changed electrical and optical properties are generated which has multi functionality in electronics devices[27-28].
2.3 Polyvinyldene fluoride (PVDF):
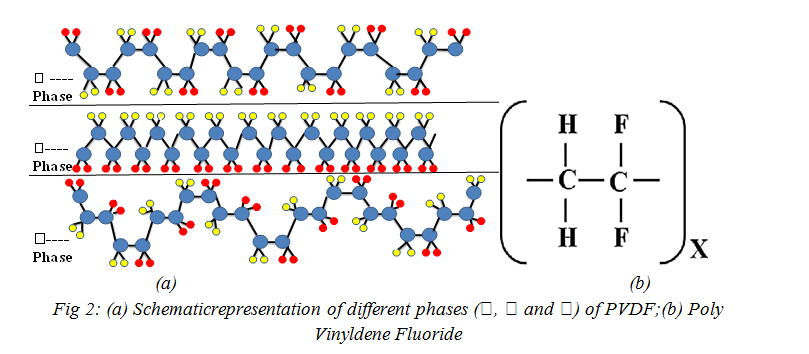
Polyvinylidene fluoride(PVDF), and its copolymers are the family of polymers with the highest dielectric constant and electro active response, including piezoelectric, pyroelectric and ferroelectric effects[29]. ‘The electro active nature is again a demanding property for wide range of functionality in medicines, generation of energy and storage, monitoring & control of ‘Sensors and Actuators’. Recent advances in the development of electro active composites allowing novel effects, such as magneto electric responses opened new applications areas [29].Semi-crystalline polymers have a complex structure and can present five distinct crystalline phases related to different chain conformations designed as all trans (TTT) planar zig-zag for the β-phase,T3GT3G(trans-gauche–trans-gauche) for the α and δ phases and T3GT3Gfor γ and ε phases [30, 32-33]. Fig. 2(a) &(b)[29,33] shows the most investigated and widely-used PVDF phases i.e. α, β and γ-phases. Each chain possesses a dipole moment perpendicular to the polymer chain. The monomer units and therefore the dipolar moments are then packed in a morphology which can show an overall dipolar contribution per unit cell as in the polar β, γ, and δ phases as shown in Fig 2(a)[30-,33].
2.4 Poly pyrrole(PPy):
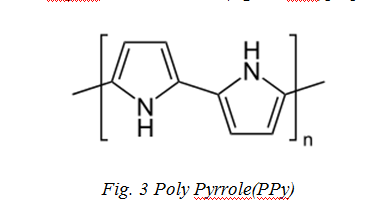
Polypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH as shown in Fig.3 [34]. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields [34-35]. Films of PPy are yellow but darken in the air due to some oxidation. Doped films are blue or black depending on the degree of polymerization and film thickness. They are amorphous, showing only weak diffraction. PPy is described as “quasi-unidimensional” vs one-dimensional since there is some cross linking and chain hopping. Undoped and doped films are insoluble in solvents but swell able. Doping makes the materials brittle. They are stable in the air up to 150 °C beyond which, the dopant starts to evolve (e.g., as HCl)[36].
PPy is an insulator, but its oxidized derivatives are good electrical conductors. The conductivity of the material depends on the conditions and reagents used in the oxidation and ranges from 2 to 100 S/cm. Higher conductivities are associated with larger anions. Doping the polymer requires that the material swell to accommodate the charge-compensating anions. The physical changes associated with this charging and discharging have been discussed as a form of artificial muscle[37]. The surface of polypyrrole films present fractal properties and ionic diffusion through them show ‘anomalous diffusion’ pattern [34-37]. PPy and related conductive polymers have two main applications in electronic devices and for chemical sensors [38].
2.5. Poly Vinyl Pyrrolidone (PVP)
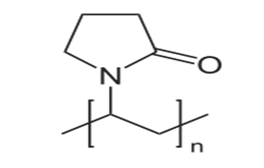
Polyvinylpyrrolidone (PVP) commonly called as polyvidone or povidone represented as shown in Fig.4[48] is a water-soluble polymer made from the monomer N-vinylpyrrolidone[39].
It is used as a binder in many pharmaceutical tablets. PVP is also used in some contact lenses and their packaging solutions. It reduces friction, thus acting as a lubricant, or wetting agent, built into the lens. Examples of this use include Bausch & Lomb’s Ultra contact lenses with Moisture Seal Technology[40-41] as a special additive for food, batteries, ceramics, fiberglass, inks, and inkjet paperto increase resolution in photo resists for cathode ray tubes (CRT)[42].
2.6. PEG/ PEO (Polyethylene glycol/ Polyethylene oxide):
Polyethylene glycol is a ‘polyether’ compound filtered out from petroleum with lots of demand applications from electronic industries to medicine world. PEG is also known as polyethylene oxide or poly oxyethylene, depending on its molecular weight. The structure of PEG is commonly expressed as H−ₙ−OH. PEG has Formula: C2nH4n+2On+1 and it is soluble in water. PEG and PEO are synonymous – they are different names for the same polymer. Historically, PEG has tended to be used to refer to polymers with a molecular mass below 20,000 g/mol, whereas PEO has been used for larger polymers, though many people use the names interchangeably or prefer one over the other.PEO polymers are used as thickeners, lubrication aids, film formers, flocculants and binders in applications including adhesives, coatings, inks, water treatment, ceramics, papermaking, agrochemicals, and electronics. Solutions of PEO have a high degree of wet tack and lubricity [43].PEO may be used to afford PEO-salt complexes which are act as polymeric electrolytes to be used for alkali metal rechargeable batteries.
2.7. Polyvinyldene fluoride) (PVDF)/Poly (methy methacrylate) (PMMA):
PVDF/PMMA blends have been studied extensively, mainly in relation to PVDF piezoelectric properties. Much attention has been paid to problems such as miscibility of the amorphous phase, crystallization of PVDF in various phases, and molecular origin of PVDF/ PMMA interactions. Moreover, blending with PMMA was described as an original way to force PVDF to crystallize into the piezoelectric phase, the effect of addition of PMMA on the crystallization and morphology of PVDF phases. Polymer blend PVDF/PMMA in different ratios are synthesized and studied. It has been discussed that ‘PVDF and PMMA’ turn into single particle, because these two are dispersed and mixed progressively due to the ‘inter diffusion’ of the initial drops of both of polymers [44,45]. The results are demonstrated by FTIR, X-ray, UV–visible, differential thermal analysis (DTA), and SEM spectroscopy [44].Characteristic absorption bands from FTIR spectrum were identified and assigned by comparison with the literature values found for PVDF/PMMA blends. The shift of C=O, observed in the carbonyl stretching frequencies of blends is due to fine interaction between the carbonyl and -CH2 groups of PMMA and PVDF respectively. The change in the UV–visible spectrum is due to complex formation which can be reflected in the form of decrease in the optical energy gap. The DTA thermograms depicts that the addition of PMMA decreased the melting temperature and the degree of crystallinity. Morphology of PMMA/PVDF blends shows crystalline domains uniformly shaped with spherulites. The morphology becomes sharper and contains a longitudinal shape note spheres for PMMA/PVDF (80/20)[44]. Numerical approach in research tuned with their experimental data represents the formation of the interface between the two particles during the ‘coalescence processes’. Besides, such work has been verified through simulation whereas numerical methods have been carried with ‘Fluent Ansys Software’. Theories too have revealed that the PMMA polymer induces a decrease of the chemical potential of the PVDF in the blends, results in a reduction of the melting point at the equilibrium. [45].
- Polymer blends with additives:
3.1 Metal Oxides Doped PPY/PVA
Metal oxides doped PPY/PVA films are developed with improved electrical, optical properties along with environmental stability and such alcohol blend thin films were synthesized using “in-situ chemical oxidative polymerization” microwave oven is required on the glass substrate to develop ‘Ammonia and Tri-methyl Ammine’—a hazardous gas sensor. These polymer nano composites materials were characterized either by structure analyses or by the measurements of conductivity taking FPT(Four Probe Technique). The surface morphology through SEM images was observed and it showed a uniform covering of the entire surface of the substrate [46].
3.2 PMMA doped with Salts
The spectral analysis in pure PMMA is compared with PMMA doped with salts which reveals that the incorporation of adipic acid (salt) to pristine PMMA, dc conductivity value of pristine PMMA increases from 5.5758×10-7 Scm-1 to 1.5233×10-6 at ambient temperature. Admittance analysis is further a cross check for the conductivity obtained from ‘conductance analysis’. ‘Magnitude Bode ploy’ shows the decline in the impedance with the addition of salt: Adipic acid to pristine PMMA [28,47]
3.3 PEO–PVP Polymer Blends Loaded with Metal Oxide ZnO:
Polymer blends PEO–PVP(50/50 wt%) loaded with metal oxide ZnO with (x = 0,1,3 &5 wt%) has enhanced dielectric properties. The porous ‘spherulites morphology’, ‘polymer-polymer’ and ‘polymer-nanoparticle’ interactions and the semi crystalline structures of these materials have been affected by the incorporation of the fillers (ZnO Nano particles) concentration in the blended polymer matrix material [48].
3.4 Poly ethylene oxide (PEO) and poly methyl methacrylate (PMMA) blend with LiClO4 (Lithium per chlorate) as dopant ionic salt, and nano particles metal oxides like ZnO, Al2O3, SiO2 and SnO2.
The electrical properties and the performance of the NSPE (Nanocomposite Solid Polymer Electrolyte) films containing PEO (polyethylene oxide) and PMMA (polymethyl methacrylate) blend in 50/50 wt% as host polymer, LiClO4(Lithium perchlorate) –dopant ionic salt, and NPs MOs with 3 wt% of ZnO, Al2O3, SiO2 and SnO2 particles as inorganic nanofillers have been investigated by “Electrochemical analyzer and precision LCR meter”. The “LSV(Linear Sweep Voltammetry)”, “CV (Cyclic Voltammetry)”, “CA (Chronoamperometry)”, and “EIS (Electrochemical Impedance Spectroscopy)” All the measurements of the above synthesised material films have been taken at surrounding (ambient) temperature. Tailoring of the dielectric and electrical behavior of these nano sized solid polymer electrolyte (NSPE) films have been done using characterisation techniques viz: DRS(Dielectric Relaxation Spectroscopy) for the frequencies ranges from 20 Hertz to1 M Hertz at: 27/35/45/55 °C for the polymer blend PEO-PMMA with 50/50wt% incorporated with salt and dopant fillers. The comparative study of structural, optical and electrical properties with different dopants (-x wt%) in PEO-PMMA and for fixed wt %( i.e. 3%) of inorganic fillers but varying temperature is studied and characteristic behavior is found and compared with the different tracings[49-50].
- Methods for Synthesis of polymer:
Different methods and techniques are one of the main objectives of this review article. Here some of the research methods for sample synthesis and their characterization techniques are presented:
4.1 Solution cast techniques:
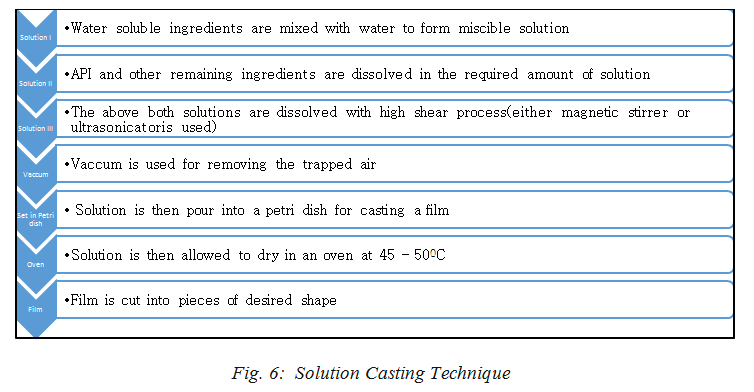
Solution casting method is based on the principle of Stokes’ law. In this, either magnetic stirrer or ultrasonicator is used with temperature control knob. In this method, polymer and pre polymer are equally merged in the suitable solution. The polymer being the matrix phase dissolved in the solution(with proper solvent)whereas, the nanoparticles, dispersed in same or different solution are allowed to be mixed through the stirrer for the specific time for proper miscibility. Fig. 6[8,13,14,15,19,30,33] shows the steps carried to prepare Polymer Nano composite films.
4.2 Dip Coating Method:
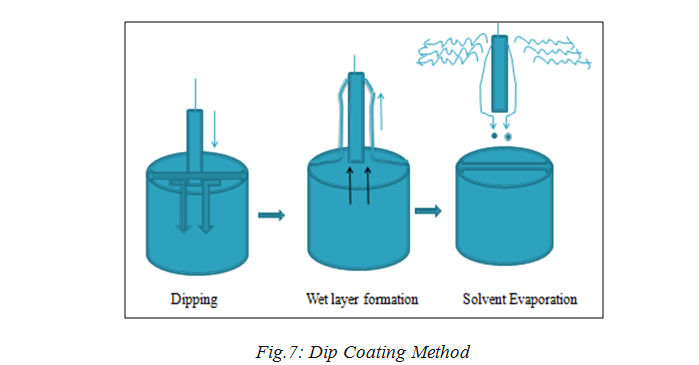
Dip coating as represented in below Fig 7[41] refers to the immersing of a substrate into a beaker containing coating material, removing the substrate from the beaker, and set it till drain. The coated piece can then be dried by force-drying or baking. It is a popular method for making thin films coated with materials along with the spin coating procedure.
4.3 Sol Gel Method:
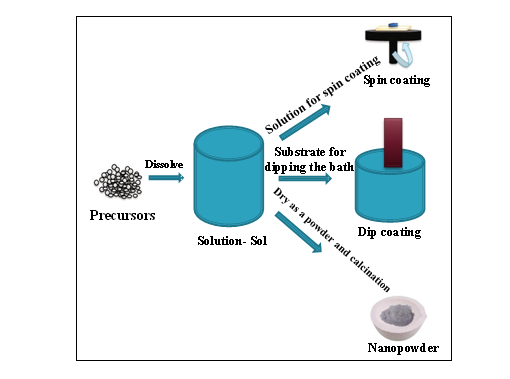
Sol Gel in materials scienceis a nice method for producing solid materials from tiny molecules. This method is especially used for the fabrication of nano tubes or MO ( metal oxides), like the oxides of Silicon(Si) or Titanium(Ti).In this technique, monomers are converted into a colloidal solution (sol), which acts as the precursor for an IN(Integrated Network or gel)for either‘ discrete particles or network polymers’. Its schematic representation is in Fig. 8[43]
- Dielectric Behavior of Polymer Blend Doped Additives/Fillers:
To characterize the optimized composition of polymer blend/gel electrolytes, different electrical and electrochemical methods such as, room temperature conductivity measurements, temperature dependence conductivity measurements, dielectric studies, modulus studies, conductance spectra, polarization techniques for measurement of ionic transport number and potential window measurements were adopted. Here some of the different polymer blends or composites are discussed with their dielectric constant, dielectric loss, conductivity, electric modulus and impedance with the help of Impedance Analyzer.
5.1 Dielectric properties of PEO–PVP Polymer Blend Loaded with Metal Oxide ZnO:
Polymer blends PEO–PVP(50/50 wt%) loaded with metal oxide ZnO with (x = 0, 1, 3 & 5 wt%) has enhanced dielectric properties[48]. The porous spherulites morphology: ‘polymer-polymer’ and ‘polymer-nano particle’ interactions and the semi crystalline structures of these materials have been significantly influenced by the concentration of the nano fillers (ZnO nano particles) in the blended polymer matrix material. Also ε՝ and ε՝՝, M՝, σ and Z spectra of these PNC materials as flexible nano dielectrics have been measured in the FR from 20 Hertz to 1 Megha Hertz by utilising the DRS(Dielectric Relaxation Spectroscopy). The DRS results at the Tambient , values of ε՝՝ increases with the increase of ZnO contents up to 3 wt% in the PEO– PVP blend matrix and deceases slightly for 5 wt%. The “Dielectric Relaxation Process” confirms the polymers “cooperative chains segmental dynamics” in the blend matrix which got raised due to the presence of ZnO NPs in the complex PNC structures. Whereas the temperature dependent study of 3 wt% ZnO containing PNC film revealed that the ε՝՝( complex permittivity) linearly increases with the increasing temperature, Also, the values of time τrelaxation and σ (electrical conductivity) obeyed the “Arrhenius behaviour” [48-50]. These materials have stability in conduction at about 3V, excellent reversibility performance (over 6V range) their significant value of ionic conductivity (~ 10–5 S cm–1) confirmed their employability for the development of solid-state rechargeable LIBs (lithium-ion batteries) as electrolyte or separator. Such these NPEs materials exhibited negative resistance region above the V(Stability Voltage) in their plots. The electrical parameters and the negative resistance properties of these NPEs were found largly affected by the crystalline nature, particle size, and dielectric constant of the nanofillers. The ionic conductivity of these NSPEs depends on the structural dynamics of inorganic nano particles incorporated in “ion-dipolar complexes” of the PNCs [51-53].
5.2 Dielectric behavior of PEO/PMMA blend with LiClO4 as dopant ionic salt and metal oxides like ZnO, Al2O3, SiO2 and SnO2 as NPs.
The electrical properties and the performance of the NSPE (Nanocomposite Solid Polymer Electrolyte) films containing polyethylene oxide and polymethyl methacrylate 50/50 wt% as host polymer, dopsant ionic salt like LiClO4(Lithium perchlorate) and NPs metal oxides of 3 wt% of MOs like: ZnO/ Al2O3/SiO2/SnO2 particles as inorganic nano fillers have been analysed by utilising the “electro chemical analyzer and precision -LCR meter”. Lowering in the values of ‘Dielectric Polarization’, high ionic conductivity values has been noted for all the NSPE films as compared to that of the without doped NSPEs films. It too has been noted that the “cooperative polymers chain segmental dynamics” got slower with the addition of NPs in the ‘ion dipolar complexes’ causes decrease in ionic conductivity of the NSPE films. The observed data and their correlation indicates that the ‘dielectric relaxation time’ and the ‘ionic conductivity’ confirmed the ions transportation which is occurs due to inter and intra-chain hopping in these polymer blends(PEO–PMMA) electrolytes. “Relaxation time” {τ(T)} and “:dc ionic conductivity” of the electrolyte films obey the “Arrhenius relation” and their activation energies exist in between 0.22eV to 0.40eV and different with the different types of NPs(Inorganic fillers) in the NSPEs. The comparative study confirmed that “the polymers chain segmental dynamics” effect the materials amorphous phase and too contributes vast applications in the lithium ions transportation and their mobility. Linear correlation in dielectric permittivity and ionic conductivity’ of these NSPEs suggested the strength of dielectric polarization, the ion transportation process in the solid ion-dipolar complexes. The ionic conductivity values of these lithium ions conducting NSPEs are ~10–5Scm–1 suggesting their suitability as flexible-type solid electrolyte materials for the design and development of dry lithium-ion batteries and several other ‘ion-conducting devices’ [49, 53].
5.3 Dielectric Property of SnO2 doped Polymer blends:
NPs incorporated polyethylene oxide/polyvinyl pyrrolidone blend matrix and PEO/PMMA–SnO2NCs were studied, and their significant data and results highlight their multi-functional applications in optoelectronics or microelectronic devices [44-48]. The SnO2 is an important n-type semiconductor oxide having wide energy band gap (~3.6 eV) [51]. SnO2 has been frequently used with different polymers for the preparation of ‘transparent organic resistive memory devices’, ‘gas sensors’, ‘transparent electrodes for solar cells’, electro chromic windows, and electrolytes for ‘energy storage/ converter devices. Furthermore, the SnO2 NPs loaded PEO matrix has also been considered as the quality performance NSPEs (NanoComposite Solid Polymer Electrolytes) as potential candidates for ‘energy storage/converter devices’ [39-40]. Literature survey unveils the structural, dielectric, and electrical properties of SnO2 dispersed in PEO- PNC matrix films over the frequency range from 20 Hz to 1 MHz at ambient temperature. The changes in the ‘degree of crystallinity of the PEO’ matrix loaded with x wt% of SnO2 and the alteration in morphology of these materials reflects its characteristics behaviour. SnO2 nanofiller concentration in these materials was kept low (≤ 5 wt%) to avoid the NPs agglomeration and successful preparation of the PNCs [ 37,39,51,54].The influenced crystallographic study of PEO-x wt% SnO2films proves a huge alteration in crystals phase concentration of the PEO due to ‘Polymer–NP’ Electrostatic Interactions. A uniform increase in the intensities of SnO2 characteristic diffraction peaks with the increase in its amount in the PEO matrix confirms the homogeneity of the NPs in the NCs which can be proved by the images obtained through SEM. The ‘Degree of Crystallinity’(In %) of the host PEO matrix nonlinearly decreases with the increase in SnO2 concentration, but there is a uniform increase in crystallinity of the ‘bulk composite material’. The FTIR results reveal weak chemical but appropriate electrostatic interactions of the SnO2 NPs with the dipolar groups of PEO chains. The dielectric and electrical properties of PEO-x wt% SnO2 films (x wt%)and x=0, 1, 3, and 5 are reported over the FR(frequency range: 20 Hz –1 MHz), at 30 °C, and also with temperature variation for the PEO-3 wt% SnO2 film as a representative sample. The dispersion of SnO2 NPs produces high IP(Interfacial Polarization) at LFR(Low Frequencies Range) and also favours the dipole ordering at HFR due to which the complex permittivity of these ‘PNC materials’ significantly increases. A weak relaxation peak was observed in the LFR of the electric modulus spectra which is corresponding to the MWS relaxation process, whereas relatively very high intense relaxation peak was appeared in the HFR(high-frequency region) attributing to the ‘conductivity relaxation’ associated with the ‘PEO chain segmental motion’. The presence of SnO2 NPs in the PEO structures increases the chain segmental dynamics and also the electrical conductivity of these NCs. The ‘dielectric permittivity’ and ‘electrical conductivity’ significantly increases with the increase in temperature of the PNC film confirming thermally activated dielectric behavior. The ‘relaxation time’ and ‘dc electrical conductivity’ of the PNC film obey Arrhenius relation with appreciably low activation energies. The enhancement of dielectric permittivity with the nanofiller concentration suggests the suitability of these materials as tunable nano dielectrics which could be promising dielectric substrates and insulators in the fabrication of flexible-type advanced.[55]
Further, PNC films in which tin oxide (SnO2) NP as nano fillers with 1, 3 and 5 wt% added with polyvinyl pyrrolidone (PVP)/polyethylene oxide (PEO) were prepared through casting of aqueous solutions. The scanning electron microscope micrographs of these PNC’s films represent the drastic changes in the ‘macrosized spherulites’ and ‘micro sized pores’ of the polymer matrix with the dispersion of different(x) wt% of SnO2 NP’s. FTIR results showed that “SnO2 NP’s behave as geometrical incarceration for the polymer blend structures due to which PEO contents crystallites anomalously changed through XRD observations. Dielectric permittivity and electrical conductivity of the materials PVP/PEO blend with different SnO2 concentration was measured in the frequency range window of 20 Hz to 1 MHz. The dielectric permittivity of these NCs were appreciably high at lower AF (Audio Frequencies) but decreased with the increase in frequency within this region and independent to frequency in the RF (Radio Frequency) region. ‘Dielectric permittivity of the developed PNC materials in the RF region revealed their principally attribution to the DP (Dipolar Polarization)’, whereas these PNCs materials are strongly influenced by the IP (Interfacial Polarization) and relaxed in the lower AF region. ‘The study of (PVP/PEO)/3wt% SnO2 film or sample confirmed that the dielectric polarization, dc electrical conductivity, and MWS relaxation are thermally active with temperature’, The nanofiller concentration dependent dielectric permittivity has reasonably low loss values of the (PVP/PEO)/x wt% SnO2 films credited their utilization as ‘tunable nano dielectrics’ for making flexible ‘biodegradable microelectronic’ components with enhanced energy storage capacitors. The lowering/decrese of τrelaxation for the high loading of SnO2conten’t in these nano composites and for the increased values of temperature of these PNC’s film affirm them as potential candidates for the advance researches in numerous activating/ conducting materials and their usages in energy harvesting and storing devices. The experimental data revealed that the (PVP/PEO)/x wt% SnO2 films are the one among the advanced-multifunctional materials having their wide applications in future intended flexible devices for optoelectronics and microelectronics[37,39-40]. These biodegrade able PNC films have multi functionality use in ‘UV- shields’, ‘optical band gap tuner’, ‘dielectric permittivity’ and ‘conductivity controllable nano dielectrics’ [51, 54, 55]. A comparative Table 1[51-52,54-55] is prepared showing the dielectric property of the SnO2 doped in different polymer blends with their scopes in market.
Table 1: Comparative Dielectric and optical behavior of SnO2 doped polymer blend
| Property | SnO2 | PEO/PMMA–SnO2 | PVP/PEO–SnO2 | PEO-SnO2 | Frequency |
| Delectric Permittivity | High | Increases with wt% ratio | Increasers with freq. |
20 Hz to 1MHz |
|
| Dielectric loss | Low | Low | |||
| Optical | Good | Non Linear increase in Absorbance | |||
| Band Gap | ~3.6eV | ||||
| Applications | Gassensors, transparentelectrodes for solar cells and electrolytesforenergy storage/ converter devices | Optoelectronics or microelectronic devices | UV- shields, optical band gap tuner, and dielectric permittivity and conductivity controllable nano dielectrics | optical band gap tuner, |
5.4 MMT Clay filled PVA/PEO polymer blend:
Organic/inorganic (NCs) of ‘polyvinyl alcohol (PVA)–polyethylene oxide (PEO)’ blend filled with montmorillonite (MMT) nanoclay (10 wt.%) were synthesized by aqueous solution casting technique. The rigorous increase of ‘dielectric relaxation time’ showed that the dispersed ‘exfoliated nanoscale MMT clay’ in the blend matrix of polymer produces a large obstacle in the ‘polymer chain dynamics’. The results obtained confirmed that the ‘real part of dielectric function’ of the NCs can be tuned by incorporating the different amount of filler (MMT clay) for their use as ‘nano dielectric materials’ in the ‘microelectronic technology [56]. Dielectric spectroscopy measures the degree of dispersion of nanoscale MMT clay in the polymer matrix and the hindrance to the polymer local chain dynamics. Ist too acts as diagnostic sensor in the development of testing and monitoring technique in the area of NC formation especially with the incorporation of MMT clay. The dielectric study revealed that the ε0 value can be tailored over the range of nearly 1 by loading the 0.5–2 wt% MMT clay in the PVA_PEO polymer blend matrix for their use as low dielectric constant nano dielectrics with improved mechanical and thermal properties in low frequency microelectronic technology. ‘The raising values in polymer chain segmental motion relaxation time with the increase of MMT clay concentration up to the 2 wt.% promote the miscibility between PVA and PEO[54,56], this is mainly due to bridging polymers chains through H-bonds with intercalated and exfoliated nanostructures. of MMT clay sheets. The consequences are that σdc conductivity of these NCs vary anomalously within the order of one in magnitude on increasing the wt ratio of MMT clay up to 10 wt%[56].
5.5 Ionic salt filled PEO /PMMA:
“The polymer blend PEO and PMMA incorporated with LiCF3SO3(Lithium Triflate) as a dopant ionic salt and PEG (Polyethylene glycol) as plasticizer prepared by ‘solution cast melt–pressed(SC) and ultrasonic(US)technique followed by microwave irradiated solution cast melt–pressed methods”. It revealed that the dielectric functions of these SPE- films anomalously vary with the sample preparation techniques and PEG content. The ionic conductivity of unplasticized electrolyte film prepared US–MW method is double as compared to the values obtained through SC technique used to prepare electrolyte films. It was found that all the ‘dielectric parameters of these electrolytes vary anomalously with the increase of PEG content and also due to the different preparation techniques for samples’ [52-53]. The ‘activation energies’ have been found from the temperature dependent values of σdc(Ionic Conductivity), “polymer segmental relaxation time and dielectric strength”. The observed data revealed that besides the ‘amorphicity’, the ionic conductivity of these electrolytes governed by the relaxation time and the dielectric strength, It too was found that the ionic conductivity of these electrolytes has correlation with the ‘dielectric strength’ and the ‘Polymer chain segmental motion relaxation time’. The ions mobility is due to cations coordinated ‘polymer chain segmental motion’ and their transportations occur by ‘hopping mechanism’. The values of ionic conductivity and relaxation time activation energies of these electrolytes are found in the range 0.22–0.33 eV. These PEO–PMMA blend electrolytes have nearly 1-2 orders of magnitude, higher ionic conductivity at T(room) as compare to the PEO/PMMA based electrolytes. Significantly, enhanced conductivity at room temperature confirms the suitability of the PEO–PMMA blend based electrolytes for the lithium-ion batteries and other electro chromic devices. ‘The unplasticized PEO–PMMA blend electrolytes structures are found amorphous due to blend miscibility and its complexations with the Li- cations[53,56-57]’.But the amorphous phase gradually falls as the PEG concentration increases in the electrolytes, which is owing to the same backbone units of PEG and PEO molecules which is coupled with segmental motion of polymers chain[53,57].
5.6 PEO–OMMT Nano Composites Film:
Structural analysis of ‘PEO–OMMT nanocomposites’ using various formalisms of dielectric processes. The significant decrease of εs at 1 wt% OMMT loading is the evidence of predominance of exfoliated OMMT structures in PEO matrix, whereas at 2 wt% OMMT the εsvalue equal to that of pure PEO film confirms the nearly equal amount of OMMT exfoliated and intercalated structures[58].The dielectric study revealed that the εs value at radio frequencies can be tuned by loading 1 to 2 wt% OMMT in the PEO matrix and synthesizing by melt compounding technique for their use as low dielectric constant nano composite materials in microelectronic technology. The ‘dielectric and electric modulus relaxation times’ confirm that in the correlated structures of nano composites, the a.c. ionic conduction relaxation is faster than that of the PEO chain segmental dynamics. The ‘hindrance to the PEO segmental motion’ due to ion-dipolar interactions with OMMT is stronger than that of the PEO interaction with Na+–MMT[57-58].The d.c. conductivity of these PEO based films vary within one order of magnitude with OMMT loading up to 10 wt%.
5.7 PMMA/n-Si substrate and Indium Tin Oxide(ITO)doped Nano Composites Film:
Thin films of PMMA (poly methyl methacrylate)were prepared on n-Si substrate and ITO(Indium Tin Oxide) glass prepared by spin coating. The breakdown field strength of PMMA is affected by adding the impurities and surface charges or the interface in the Si substrate[52, 60, 67].PMMA dielectric films with “MIS and MIM” structures are obtained by ‘spin coating technique’. The XRD spectroscopy represents it’s (PMMA) amorphous nature. The dielectric constant of the two structured PMMA obtained through the HF(high frequency), C-V electrical characterization for both is similar i.e. 3.9 at 1 M Hz. The ‘impurities’ and ‘surface charges’ or ‘interface states’ existed in the Si-substrate may contribute to the lowering of the breakdown voltage of PMMA. And the leakage ‘current density’ of PMMA is about 10-6 A cm-2. The transmittance spectra shows that the transmittance of PMMA/ITO glass is above 80%, and PMMA improves the transparency of this structure in the visible range [58,66].
5.8 Metal oxides doped PPY-PVA blend thin films:
The pyrrole (monomer) was double distilled prior to use [46]. The polyvinyl alcohol and pyrrole metal oxide doped films are prepared[46,52]. It was observed that the polymer blend PPY/PVA is composed of mixed phase i.e. conducting and insulating of the polymer. All these UV –visible spectral data clear that in these synthesized metal oxides doped PPY-PVA formation take place. Further increase of the particles.
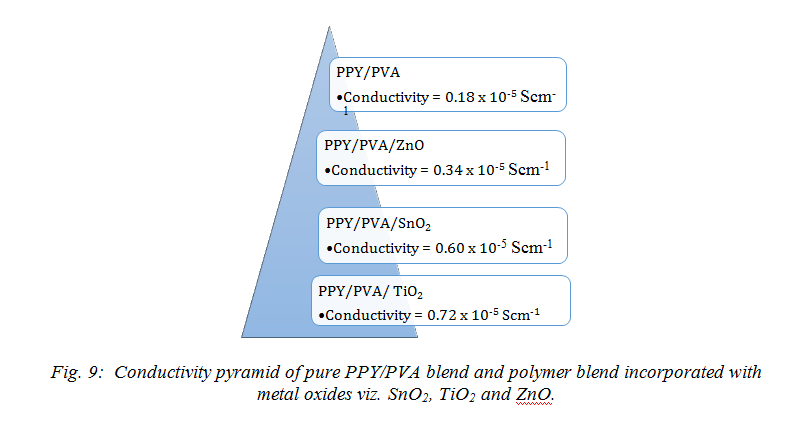
(above the percolation threshold) in the blends results in improvement of the conducting network and hence enhance the conductivity of the blend increases by dopant of metal oxide TiO2, ZnO and SnO2 doped PPY-PVA blend thin film. It is interesting to notice that, despite the insertion of metal oxide dopant and insulating PVA, the DC conductivity of the PPY-PVA blend increases and it too was found to be significantly higher than the PPY–PVA films at room temperature. In Fig. 9 the pyramid represents [46-48, 51-52, 54, 67, 68] increasing conductivity withdifferent metal oxide nanofillers.
Conclusions:
In this review, we have discussed the recent progress of polymer or polymer blend loaded with metal oxides or salts nano composites (PNCs). Techniques related to the synthesis of films or pellets have been widely reviewed, from the diverse doped polymer blends synthesis techniques. Synthesis techniques and dielectric properties and semiconducting characteristics of diverse synthesized polymer with salts, SiO2, CuO, SnO2, ZnO, Fe2O3, TiO2, MMT and OMMT were mentioned in details which were synthesized in diverse nanostructures such as NPs, NWs, nano platelets, NCs, NTs, and NFs. It is observed that different synthetic strategies were taken and explored to enhance dielectric efficiency, including metal oxides doping, filling with inorganic materials and salts. The structural properties of these synthesized polymer nano composites have also been discussed herein. Their enhanced dielectric properties with less dielectric losses with the addition of metal oxides indicates conducting nature and reflects its wide applications in the field of microwave electronics or optoelectronics. Notably, several studies argued the development of multifunctional PNCs, but it too was noticed that few got successful response in conducting materials depending on the band gaps between conduction and valence bands whereas few of them have wide use in insulators. To overcome the limitation in several blends different approaches including doping of other materials (Salts, SiO2, CuO, SnO2, ZnO, Fe2O3, TiO2, MMT and OMMT) have been proposed and reviewed here. It can be concluded that further progress and development of new polymer blends filled with new dopants would facilitate promising applications in opto-electronics devices. Further advancement over coming limitations of MO based polymer blends, as mentioned above, would lead these films be more attractive candidate material, for future electronics. Also, these PNCs would participate in fascinating multidisciplinary research such as bioelectronics, opto electronics, new frequency generators and degradable electronics, soft robotics, and epidermal electronics. In view of the above mention results in the present review article, it can be inferred that all the optimized composition of polymer blend/gel electrolytes using inorganic materials or given salts can be used as a potential candidate as an electrolyte materials in different electrochemical devices. Ionic liquid based polymeric systems have to be further investigated in order to improve their electrochemical potential window, which can further improve the device performance [51, 54-55, 56-59, 68].
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References:
- Bruce, D. W., O’Hare, D., and Walton, R. I. (2011), “Energy Materials”, John Wiley& Sons, Chichester, UK.
- Fenton, D. E., Parker, J. M., and Wright, P. V. (1973), “Complexes of alkali metal ionswith poly (ethylene oxide)”, Polymer, vol. 14, no. 11, pp. 589-589.
- Gray, F. M. (1991), “Solid Polymer Electrolytes: Fundamentals and Technological, Applications”, VCH Publishers, New York.
- Kumar, D., and Hashmi, S. A. (2010), “Ionic liquid based sodium ion conducting gel polymer electrolytes”, Solid State Ionics, vol. 181, no. 8-10, pp. 416-423.
- Ohno, H. (2005), “Electrochemical aspects of ionic liquids”, Wiley, New Jersy.
- Thakur, A. K., and Hashmi, S. A. (2010), “Polymer matrix-filler interaction mechanism for modified ion transport and glass transition temperature in polymer electrolytes composite”, Solid State Ionics, vol. 181, no. 27-28, pp. 1270-1278.
- Tripathi, S. K., Jain, A., Gupta, A. and Mishra, M. (2012), “Electrical and electro chemical studies on magnesium ion based polymer gel electrolytes”, Journal of Solid State Electrochemistry, vol. 16, no. 5, pp. 1799-1806.
- Karin Moller,ThomasBein, and Reinhard X. Fischer (1998), “Entrapment of PMMA Polymer Strands in Micro- and Mesoporous Materials”, Chem. Mater. 10, 1841-1852.
- Jolly Bhadra, Asma Alkareem, Noora Al-Thani, Journal of Polymer Research (2020), “A review of advances in the preparation and application of polyaniline based thermoset blends and composites” 27: 122.
- Gao X-Z, Liu H-J, Cheng F, Chen Y (2016) Thermo responsive polyaniline nanoparticles: preparation, characterization, and their potential application in waterborne anti corrosion coatings. Chem Eng J 283:682–691. https://doi.org/10.1016/j.cej.2015.08.015
- Wu X, Lu C, Xu H, Zhang X, Zhou Z (2014) BiotemplatesynthesisofPolyaniline@cellulose Nano whiskers/natural rubber Nanocomposites with 3D hierarchical multi scale structure and improved electrical conductivity. ACS Appl Mater Interfaces 6(23):21078–21085. https://doi.org/10.1021/am505924z
- Borsoi C, Zattera AJ, Ferreira CA (2016) Effect of cellulose nano whiskers functionalization with polyaniline for epoxy coatings. Appl Surf Sci 364:124–132. https://doi.org/10.1016/j.
apsusc.2015.12.140.
- Shabani-Nooshabadi M, Ghoreishi SM, Jafari Y, Kashanizadeh N(2014) Electrode position of polyaniline-montmorrilonite nanocomposite coatings on 316L stainless steel for corrosion prevention J. Polym Res 21(4):416. https://doi.org/10.1007/s10965-014-0416-5
- Qiang Z et al (2014) The dielectric behavior and origin of high-k composites with very low percolation threshold based on unique multi-branched polyaniline/carbon nanotube hybrids and epoxy resin. Compos Part A Appl Sci Manuf 64:1–10
- Tsotra P, Friedrich K (2004) Short carbon fiber reinforced epoxy resin/polyaniline blends: their electrical and mechanical properties. Compos Sci Technol 64 (15): 2385–2391.https://doi.org/10.1016/j.compscitech.2004.05.003.
- Rong G, Zhou D, Pang J (2018) Preparation of high-performance anti fouling polyphenyl sulfone ultra filtration membrane by the addition of sulfonated polyaniline. J Polym Res 25(3):66. https://doi.org/10.1007/s10965-018-1463-0
- Jlassi K, Chandran S, Poothanari MA, Benna-Zayani M, Thomas S, Chehimi MM (2016) Clay/Polyaniline hybrid through Diazonium chemistry: conductive Nanofiller with unusual effects on interfacial properties of epoxy Nano composites. Langmuir 32(14):3514–3524. https://doi.org/10.1021/acs.langmuir.5b04457
- Gu H et al (2013) Flame-retardant epoxy resin Nano composites reinforced with Polyaniline-stabilized silica nanoparticles. Ind Eng Chem Res 52(23):7718–7728. https://doi.org/10.1021/
ie400275n.
- Hu C, Li Y, Kong Y, Ding Y (2016) Preparation of poly(o-toluidine)/nano ZnO/epoxy composite coating and evaluation of its corrosion resistance properties. Synth Met 214:62–70. https://doi.org/10.1016/j.synthmet.2016.01.021.
- Chevalier JW, Bergeron JY, Dao LH (1992) Macro molecules25(13): 3325–3331. “Synthesis, characterization and properties of poly(N-alkylanilines)”.
https://doi.org/10.1021/ma00039a001
- Schomburg KC, McCarley RL (2001) Surface-confined monomerson electrode surfaces. 11. Electrochemical and infrared spectroscopic characteristics of aniline-terminated Alkanethiol mono layers on an electrochemically treated in non aqueous media. Langmuir 17(6):1993–1998. https://doi.org/10.1021/la0010222
- Kathirgamanathan P (1993) Curable electrically conductive resins with polyaniline fillers. Polymer (Guildf) 34(13):2907–2908.https://doi.org/10.1016/0032-3861(93)90141-V.
23.Rawat NK, Pathan S, Sinha AK, Ahmad S (2016) Conducting poly(o-anisidine) nano fibre dispersed epoxy-siloxane composite coatings: synthesis, characterization and corrosion protective performance. New J Chem 40(1):803–817. https://doi.org/10.1039/C5NJ02295A.
- Raheem GaayidKadhim(2016), “Study of Some Optical Properties of Polystyrene – Copper Nanocomposite Films”, World Scientific News 30 14-25, EISSN 2392-2192.
- A. Tawansi , A. El-khodary , H.M. Zidan , S.I. Badr (2002), Poly test Material Behavior, 21 / 381–387 “The effect of MnCl2 filler on the optical window and the physical properties of PMMA Polymer Testing films”,
- Chitra S, Mahalakshmi P, Dr. Radha KP , “Vibrational and Impedance analysis of polymer electrolyte based on PMMA complexed with adipic acid”, International Journal of Multi disciplinary Education and Research ISSN: 2455-4588; Impact Factor: RJIF 5.12www.multieducationjournal.comVolume 1; Issue 4; June 2016; Page No. 15-18.
- A.F. Mansour, S.F. Mansour and M. A. Abdo (2015), “Improvement Structural and Optical Properties of ZnO/ PVA Nanocomposites”, IOSR Journal of Applied Physics (IOSR-JAP) e-ISSN: 2278-4861.Volume 7, Issue 2 Ver. II, PP 60-69 www.iosrjournals.org DOI: 0.9790/4861-07226069.
28.Paula Obreja, Dana Cristea, MunizerPurica, Raluca Gavrila, Florin Comanescu (2007) POLIMERY, 52, nr 9, Pg: 679., “Polymers doped with metal oxide nanoparticles with controlled refractive index”, National Institute for Research and Development in Micro technologies, .
- P. Martins, A.C. Lopes, S. Lanceros-Mendez(2014) Progress in Polymer Science, 39,683-706, “Electroactive phases of polyvinylidene fluoride(PVDF):Determination, processing and applications”.
- Salimi A, Yousefi AA(2003),“FTIR Studies of beta-phase crystal formation in stretched PVDF films”. Polymer Testing 22:699–704.
- Chang YM, Lee JS, Kim KJ(2007) Solid State Phenomena 2007;124:299–30,“Heartbeat monitoring technique based on corona-poled PVDF film sensor for smart apparel application”, 2.
- Kepler RG, Anderson RA(1978) Journal of Applied Physics,49:4490–4,“Piezoelectricity and pyro electricity in polyvinylidene fluoride”,.
- Lovinger AJ(1982) Macromolecules 15:40–4.,“Annealing of poly(vinylidene fluoride) and formation of a fifth phase”.
- Ahmad Sharifi-Viand, Diffusion through the self-affine surface of polypyrrole filmVacuum, doi:10.1016/j.vacuum.2014.12.030.
- Sharifi-Viand, Ahmad (2012) Journal of Electro analytical Chemistry,671:51–57. ,”Investigation of anomalous diffusion and multi fractal dimensions in polypyrrole film”. doi:10.1016/j.jelechem.2012.02.014.
- Vernitskaya, Tat’Yana V.; Efimov, Oleg N. (1997), Russ. Chem. Rev. 66 (5):443–51-57. Bibcode:1997RuCRv..66..443V, “Polypyrrole: a conducting polymer; its synthesis, properties and applications”, doi:10.1070/rc1997v066n05abeh000261.
- Baughman,RayH.(2005),”Playing Nature’s Game with Artificial Muscles”. Science, 308 (5718):63–65. doi:10.1126/science.1099010. PMID15802593. S2CID180181717.
- Janata, Jiri; Josowicz, Mira (2003), Nature Materials. 2 (1):19– 24, “Progress Article: Conducting polymers in electronic chemical sensors”. doi:10.1038/nmat768. {PMID12652667. S2CID1250380}.
- Haaf, F.; Sanner, A.; Straub, F. (1985,. Polymer Journal. 17: 143–152.“Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses”. doi:10.1295/polymj.17.143.
- Bühler, Volker (2005), Heidelberg, New York: Springer. pp. 1–254. “Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone”, Berlin, doi:10.1007/b138598. ISBN978-3540234128.
- Sarat Kumar Sahoo,Narendiran Sivakumar(2018), Science Direct, Elsevier Pg. 1-24,“Perovskite Photovoltaics : Basic to advanced Concepts and Implementation”.
- Swei, J.; Talbot, J. B. (2006). “Development of high-definition aqueous polyvinylpyrrolidone photoresists for cathode ray tubes”. Journal of Applied Polymer Science. 102 (2): 1637–1644. doi:10.1002/app.23950
- Hidenori Nakamura, Yasushi Matsui(1995), Journal of American Chemical Society, 117,9, 2651-2652,”Silica Gel Nanotubes obtained by Sol Gel Method, https://doi.org/10.1021/ja0014a031.
- I.S. Elashmawi, N.A. Hakeem (2008), Polym. Eng. Sci. 48 -895–901, “Effect of PMMA addition on characterization and morphology of PVDF”.
- Sara Aida, Anissa Eddhahaka, Sofiane Khelladib, Zaida Ortegac, Sana Chaabania, Abbas Tcharkhtchia(2020), HAL Id: hal-02466281, “Miscibility of PVDF/PMMA polymer blends: thermodynamics, experimental and numerical investigations”,
https://hal.archives-ouvertes.fr/hal-02466281.
- D. B. Dupare, D. Shirsat, A. S. Aswar(2009), “Metal Oxides Doped PPY-PVA Blend Thin Films Based Gas Sensor”, Sensors and Transducers, ISSN 1726-5479,© 2009 by IFSA.
- Qais M. Al-Bataineh, Ahmad.A. Ahmad, A.M. Alsaad, Ahmad D. Telfah(2021), “Optical characterizations of PMMA/metal oxide nanoparticles thin films: band gap engineering using a novel derived model”, Heliyon 7,e05952.
- ShobhnaChoudharya(2018), “Structural, optical, dielectric and electrical properties of(PEO–PVP)–ZnO nanocomposites”, Journal of Physics and Chemistry of Solids, DOI: 10.1016/j.jpcs.2018.05.017.
- Priyanka Dhatarwal, Shobhna Choudhary, R.J. Sengwa(2018), Composites Communications 10, Pg: 11–17 “Electrochemical performance of Li+ – ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nanoparticles for the lithium ion batteries.
50.Shobhna Choudhary, R. J. Sengwa(2017), Electrochimica Acta, EA 29868, “Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes”, DOI: http://dx.doi.org/doi:10.1016/j.electacta.2017.07.051.
- Choudhary S (2017), Compos Commun 5:54–63, “Structural and dielectric properties of (PEO–PMMA)–SnO2 nanocomposites”.
52 Orlandi MO (2020), Elsevier Inc.Amsterdam, “Tin oxide materials: synthesis, properties, and applications”.
- R. J. Sengwa, Priyanka Dhatarwal, Shobhna Choudhary(2014), Electrochimica Acta, “ Role of preparation methods on the structural and dielectricproperties of plasticized polymer blend electrolytes: Correlation between ionic conductivity and dielectric parameters”, http://dx.doi.org/10.1016/j.electacta.2014.07.120.
- Priyanka Dhatarwal, ShobhnaChoudhary,R. J. Sengwa(2020),Polymer Bulletin “Significantly enhanced dielectric properties and chain segmental dynamics of PEO/SnO2 nanocomposites”, https://doi.org/10.1007/s00289-020-03215-2.
- Priyanka Dhatarwal, R.J. Sengwa, Shobhna Choudhary(2020), Optik – International Journal for Light and Electron Optics 221, 165368, “ Multifunctional (PVP/PEO)/SnO2 nano composites of tunable optical and dielectric properties”.
- Sengwa RJ, Choudhary S, Sankhla S (2010), Comps Sci Tech 70:1621–1627, “Dielectric properties of montmorillonite clay filled poly(vinyl alcohol)/poly(ethylene oxide) blend nanocomposites”.
- Choudhary S., Sengwa RJ (2015), Polym Bull 72:2591–2604, “Dielectric dispersion and relaxation studies of melt compounded poly(ethylene oxide)/silicon dioxide nanocomposites”.
- Sengwa RJ, Choudhary S (2017), J Alloys Compd 701:652–659, “Dielectric and electrical properties of PEO–Al2O3 nanocomposites”.
- Choudhary S, Sengwa RJ (2017), J Polym Res 24:54, “Morphological, structural, dielectric and electrical Properties ofPEO–ZnO nanodielectric films”.
- Sengwa RJ, Choudhary S, Dhatarwal P (2019), Adv Compos Hybrid Mater 2:162–175,“Investigation of alumina nanofiller impact on thestructural and dielectric properties of PEO/PMMA blend matrix-based polymernanocomposites”.
- Dhatarwal P, Sengwa RJ (2019),JPolym Res 26:196,“Impact of PVDF/PEO blend composition on the β-phase crystallizationand dielectric properties of silica nanoparticles incorporated polymer nanocomposites”.
- Choudhary S, Sengwa RJ (2019), J InorgOrganometPolym 29:592–607, “Investigation on structural and dielectric properties of silica nanoparticlesincorporated poly(ethylene oxide)/poly(vinyl pyrrolidone) blend matrix based nanocomposites”.
- Sengwa RJ, Choudhary S, Dhatarwal P (2019), J Mater Sci: Mater Electron 30:12275–12294,“Nonlinear optical and dielectric properties of TiO2 nanoparticles incorporated PEO/PVP blend matrix based multifunctional polymer nanocomposites.
- Choudhary S, Sengwa RJ (2018) ,CurrAppl Phys 18:1041–1058, “ZnO nanoparticles dispersed PVA–PVP blend matrix based high performance flexible nanodielectrics for multifunctional microelectronic devices”.
65.Shobhna Choudhary, . R. J. Sengwa,, Bull. Mater. Sci., Vol. 35, No. 1, February 2012, pp. 19–25, Indian Academy of Sciences, “Dielectric properties and structural dynamics of melt compounded hot-pressed poly(ethylene oxide)–organophilic montmorillonite clay nanocomposite films”.
- H Q Zhang, Y Jin and Y Qiu (2015), Materials Science and Engineering 87 – 012032 , “The
enhanced optical and electrical characteristics of PMMA film prepared by spin coating method”, doi:10.1088/1757-899X/87/1/012032.
- Fenton(1973), F al. developed the first ion conducting polymer electrolyte based on
polyethylene oxide (PEO) which was dissolved with alkali metal salts.
- Yeosang Yoon, Phuoc Loc Truong, Daeho Lee, and Seung Hwan Ko(2011), Nano science: An open access journal “Metal-Oxide Nanomaterials Synthesis and Applications in Flexible and Wearable Sensors”
