| Abstract:
Considering liposomes are just one of numerous drug delivery systems that can be used to deliver medication to a specific area, we explored them in this review article. Liposomes are small synthetic vesicles with a spherical form that can be made using cholesterol and organic phospholipids. Vaccination is the most effective method of preventing infectious diseases and saving lives. The development of lipid nanoparticles and liposomes as subunit vaccines for infectious diseases has undergone substantial research and development. Positively charged lipid-bilayer vesicles known as cationic liposomes are a brand-new and fascinating adjuvant approach that has just lately come back into fashion. The potential adjuvants for vaccinations include cationic liposomes. Key word: Liposomes, cationic liposomes, phospholipid, vaccination.
|
Introduction:
During the diffusion of phospholipids in water, they develop a closed structure naturally, with phospholipid bilayer membranes enclosing an external aquatic environment. The term “liposome” refers to this vesicular system [1]. The microscopic, spherical vesicles known as liposomes can be made from a variety of substances, containing membrane proteins, lengthy saturated fats, phosphatidylcholine, sphingolipids, cholesterol, and non-toxic surfactant [2]. liposomes are basically used to improve the solubility and permeability of hydrophobic and hydrophilic drugs. When particular lipids are hydrated in aqueous conditions, liposomes spontaneously form as Colloidal or microparticulate carriers typically 0.05 to 5.0 m in diameter [3]. The material that composes liposomes is generally both biocompatible or biodegradable and They include an aqueous material that is surrounded by more than one lipid bilayer made of natural or synthetic materials [4]. Immunotherapy and vaccination work to combat diseases by utilizing the host immune system, which is triggered by the antigenic components (also known as antigens) of disease-causing organisms (also known as pathogens). This immunity can then be used to eradicate infections with the same antigens (Ages) [5].
The key information about pandemic threats as well as their negative socioeconomic consequences for individual states and the global economy demonstrates. The importance of creating safe and efficient methods for treating and preventing viral infections [6]. Traditional vaccines contain live organisms that which is dead or diminished. When developing a vaccination using liposomes, it is important to consider the particle’s net charge, and these particles are frequently either negative (anionic liposomes) or positive (cationic liposomes) [7]. As a vaccine adjuvant, liposomes were tested on humans for the first time, A malaria protein that had been synthesized was encased in multilamellar liposomes that also included cholesterol, neutral and anionic saturated phospholipids, and monophosphorylate (MPLA) as a lipid A adjuvant [8].
- Classification of liposomes:
Liposomes are categorized according to
- Structure
- preparation technique
- Composition and application
- Standard liposomes
- Special liposomes
- Classification based on the type of Structure: [9]
| Type | Size | No. of layer | abbreviation |
| Large unilamellar vesicle | < 100 nm | One | LUV |
| Small unilamellar vesicle | 20 -100 nm | One | SUV |
| Unilamellar vesicle | All size range | One | UV |
| Giant unilamellar vesicle | < 1 mm | One | GUV |
| Oligolamellar vesicle | 0.1-1 mm | Approx. 5 | OLV |
| Multi vesicular vesicle | More then1 mm | Multiple | MV |
| Multilamellar vesicle | More then 0.5 | 5-25 | MLV |
Table no 1: Classification based on the structure
- Classification based on the type of Preparation Technique: [10-14]
- Vesicle prepared by RPE method
- Multi lamellar vesicle made by RPE method
- Dried reconstituted vesicle
- Frozen multi-lamellar vesicle
- Vesicle prepared by extrusion technique
- Dehydration- Rehydration method
- Classification based on the type of composition and application:
| Sr no | Types of liposomes | Composition | Ref no |
| 1 | Standard liposomes | Neutral and negatively charged phospholipid & cholesterol | 9 |
| 2 | Fusogenic liposomes | Reconstituted sendai virus envelops | 16 |
| 3 | PH complex liposomes | Phospholipid such as PER & DOPE | 17 |
| 4 | Positively charged liposomes | Cationic lipid with DOPE | 18 |
| 5 | Extended circulatory liposomes | CL or CLC attached with monoclonal antibody | 9 |
Table no 2: Classification based on composition & application
- Classification based on the type of Standard liposomes: [19]
- Stabilize natural lecithin (PC) formulations
- Glycolipids containing liposome
- chain phospholipids
- Classification based on the type of special liposomes: [9]
- Bipolar fatty acid
- Antibody directed liposome.
- Methyl/ Methylene x- linked liposome.
- Lipoprotein coated liposome.
- Carbohydrate coated liposome.
- Multiple encapsulated liposome
- Advantages of liposomal DDS: [ 11,12,19]
- Can be produced in various sizes.
- Increased therapeutic effectiveness.
- Cancers can be targeted with specific passive targeting.
- Enhanced pharmacokinetic action.
- It lowers the toxicity of encapsulated drug.
- There are several different ways to administer it.
- Both positively and negatively charged compounds have interaction capacity with liposomes.
- The DNA is sometimes protected from damaging processes by liposomes.
- Targeting specific tissues or cells with liposomes is possible.
- Disadvantages of liposomal DDS: [11,12,19]
- It is possible to use solar energy during the day.
- The production costs are high.
- Leaking drug encapsulation while being stored.
- It is unable to be used for rare diseases.
- Phospholipid sometimes undergoes reactions like oxidation and hydrolysis.
- Liposomes having a short half-life.
- Liposomes also having low solubility.
Method of preparation:
- General method of preparation of liposomes [20]
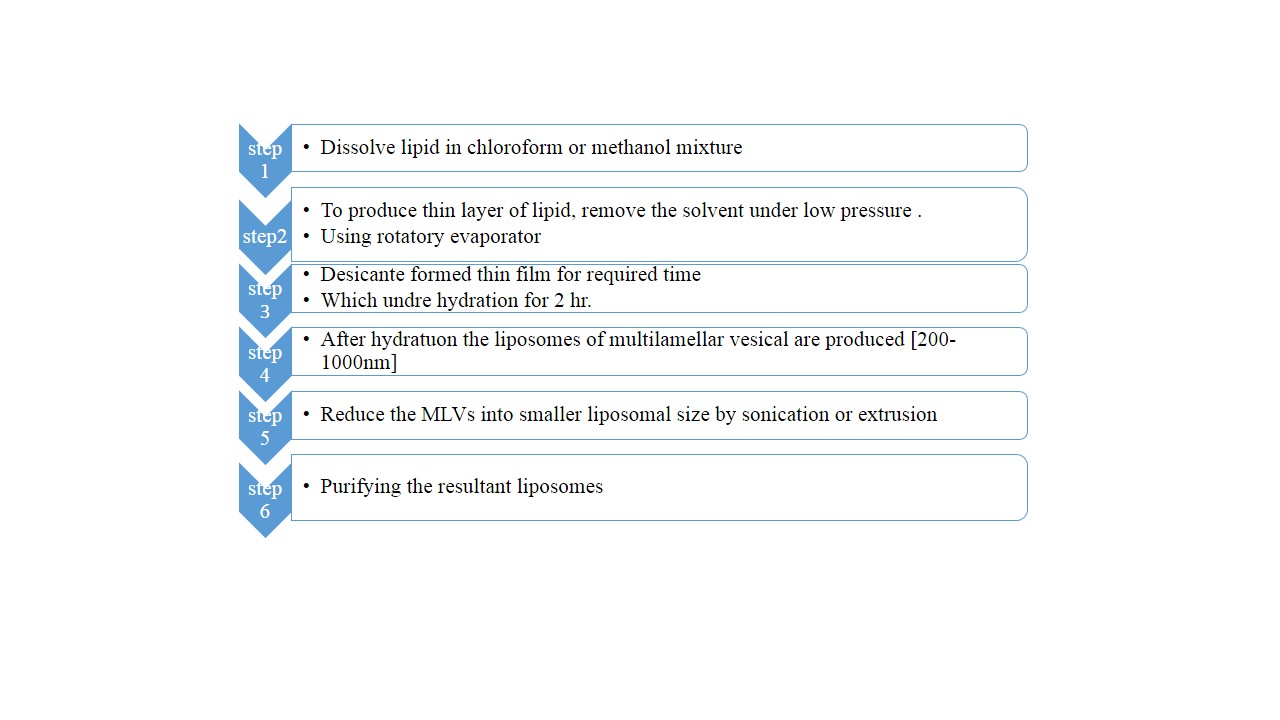
Fig no 1: General method of preparation
- Specific method of preparation: [21,22,23]
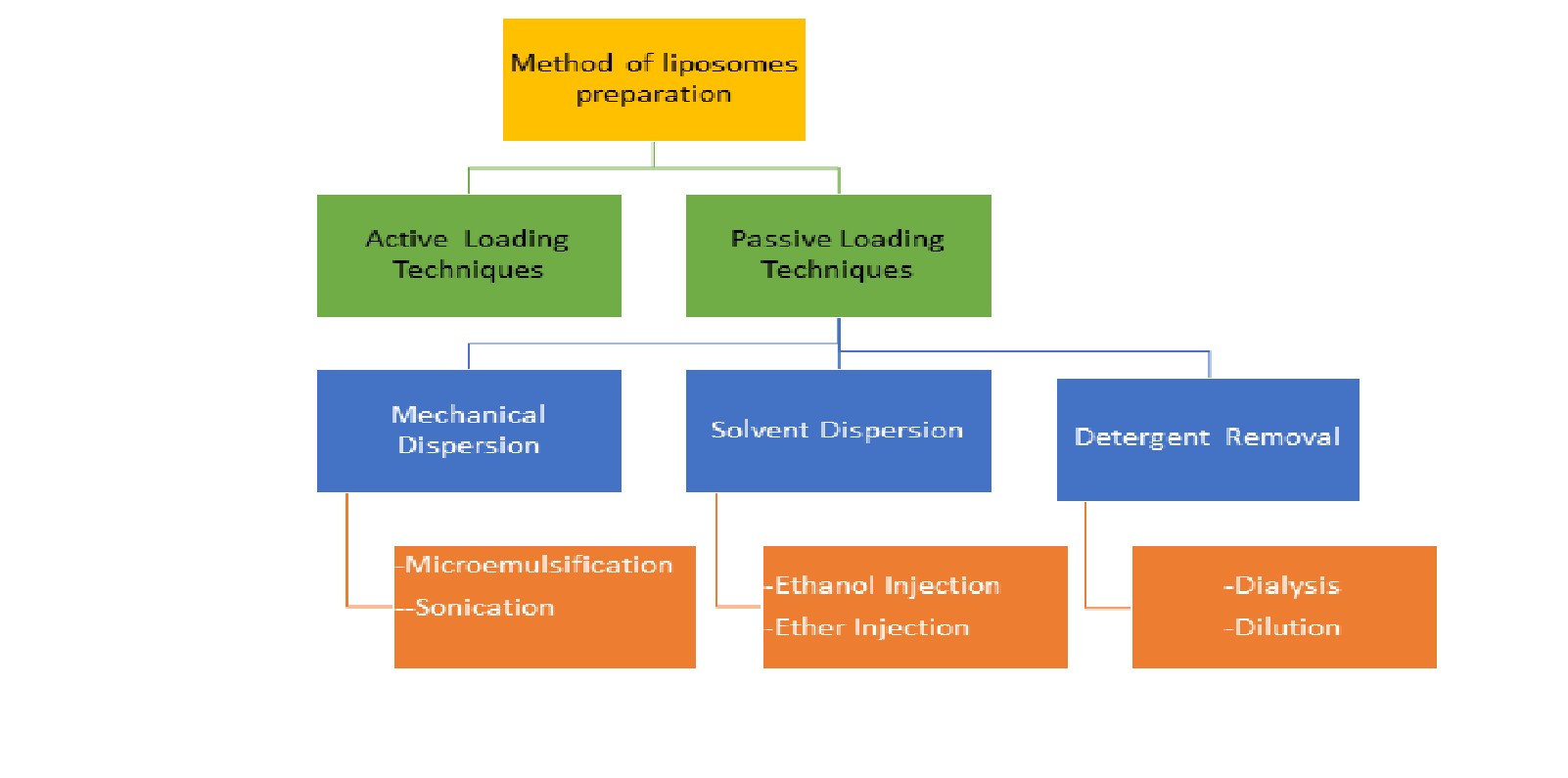
Fig no 2: Specific Method of Preparation
- Passive loading technique:
Different methods for producing liposomes result in different physicochemical properties, which affect how well they perform in vitro (for sterilization and shelf life) and in vivo (for disposition) [22]. Passive loading techniques are classified into 3 main Types which are based on the dispersion technique
- Mechanical Dispersion Method
- Solvent Dispersion Method
- Detergent Removal Method
- Mechanical Dispersion:
- A:Sonication:
The SUVs manufacturing process with a diameter of between 15 and 25 um is done using this method. Multi lamellar vesicle are sonicated using maybe a probe sonicators or a bath sonicators in inert environment. While bath sonicators are used for large volumes, when small amounts of high energy are needed, probe sonicators are used [23]. The main issues with this technique include its extremely low internal volume/encapsulation efficacy, potential phospholipid and chemical degradation [24].
- Solvent Dispersion:
- A:Ether Injection:
This method is also known as solvent vaporization. between 55 and 65 degrees or when there is low pressure. Lipids in a mixture of any such methanol or ethanol and the diethyl-ether solution are progressively infused into a liquid form of the substance that needs to be contained. As a result, liposomes are produced as a result of removing the ether under a vacuum. The population’s heterogeneity represents the technique’s main problem (70 to 200 nm) and the chemicals that must be sealed off exposure to the organic chemicals at high temperatures [25].
- B:Ethanol Injection:
Immediately injected into a large buffer excess is an ethanol-lipid solution. MLVs instantly begin to develop. The method has a number of drawbacks, including a population that they are extremely diluted liposomes that have a size range of 30 to 110 nm., ethanol that forms into an azeotrope with water that makes it difficult to remove completely, and a high likelihood that different macromolecules with biological activity will inactivate when even small quantity of ethanol [26].
- Detergent Removal:
- A:Dialysis:
At the necessary concentrations of microspheres, detergents have successfully solubilized lipids (CMC). The micelles get better and better at phospholipids when the detergent is withdrawn, eventually combining to form LUVs. Dialysis was used to eliminate the detergents. For the elimination of detergents, a commercial product known as Lipoprepration a dialysis system variant is available. Equilibrium dialysis can be carried out using dialysis bags filled with sizable, detergent-free buffers [27].
- Liposomal based drug available in market:
| Sr no | Product | Drug | Administration | Composition | Indication | Year approved | Ref no |
| 1 | Ambisome | Amphotericin B | Intravenous | HPSC, DSPG, cholesterol & amphotericin B | Fungal infection | 1990 | 28 |
| 2 | Abelcet | Amphotericin B | Intravenous | DMPC & DMPG in 7:3 molar ratio | Fungal infection | 1996 | 29 |
| 3 | DepoCyt | Morphine sulfate | Epidural | DOPC, DPPG, cholesterol | Lymphomatous | 1995 | 30 |
| 4 | Doxil | Doxorubicin | Intravenous | HPSC: cholesterol: PEG 2000-DSPE {56:39:5 RATIO} | Breast cancer | 1990 | 31,32 |
| 5 | depoDur | Morphine sulfate | Epidural | DOPC, DPPG, cholesterol and triolein | Pain | 2004 | 33 |
| 6 | Marqibo | Vincristine sulfate | Intravenous | Liposome Egg sphingomyelin and cholesterol | Acute lymphoblastic leukemia
|
2012 | 34 |
| 7 | Visudyne | Verteporfin | intravenous | EPG and DMPC | Age-related molecular degeneration
|
2000 | 35,36 |
Table no 3: Liposomal-based drug available in market
- Vaccines:
Many vaccinations are created using weakened or dead viruses, which allow antibodies to attach and kill live viruses [37]. The most important medical treatment when battling infectious infections is vaccination [38]. The invention by Edward Jenner in 1796, vaccinations have eradicated infectious diseases like hepatitis, malaria, smallpox, diphtheria, and influenza on a global scale. This was accomplished at the end of the 18th century by using the smallpox prevention properties of clinically mild cowpox infections [39]. The person who has received the vaccination becomes immune to the disease on subsequent exposure, and the pathogen-induced sickness’s undesirable side effects are avoided. Effective immunizations have largely controlled many infectious illnesses that previously caused major young children’s morbidity and mortality [40].
- Vaccine adjuvant:
The word “adjuvant” originates from the Latin word “adjuvaer” which means “to help” or “to enhance.” [41]. Recent developments in vaccine development have made it possible to create vaccinations that are extremely pure, safe, and straightforward. Adjuvants are components that can improve and/or modify immune responses that are specific to an antigen related to vaccines [42]. Modern vaccines can now be built on recombinant antigens with better safety profiles that have been logically designed and contain highly purified components. Accidentally, the immune-boosting abilities of an adjuvant added to a vaccination were discovered much like many other medical discoveries [43]. Adjuvants are necessary in vaccinations with poor immunogenicity antigens even if Vaccines made using intact (or dead) viruses or bacteria have a built-in immunity to disease (e.g., peptides, small haptens) [37]. For around 70 years, the only adjuvant permitted for use in certified vaccinations was aluminium, which was first used in human immunizations in 1932 [44]. Aluminium adjuvants are excellent for vaccinations targeting diseases destroyed mostly by antibodies since they work primarily to boost antibody formation. Intracellular pathogen-related infections have not been prevented by aluminium-adjuvanted vaccines [45]. The selection of adjuvant and formulation it may depend on a variety of variables, such as the physicochemical characteristics of the vaccination antigen, the desired type of immune response, the target’s age population, and the method of vaccination [46]. Typically, immunisation with pure protein antigens produces a minimal T cell response and a minor antigen activity. To prevent wasting potentially effective vaccination antigen candidates, Adjuvant choice must be considered while choosing the vaccine antigen. Immunotherapy and vaccination work to combat diseases by utilising the host immune system, which is triggered by the antigenic components (also known as antigens) of disease-causing organisms (also known as pathogens) [47].
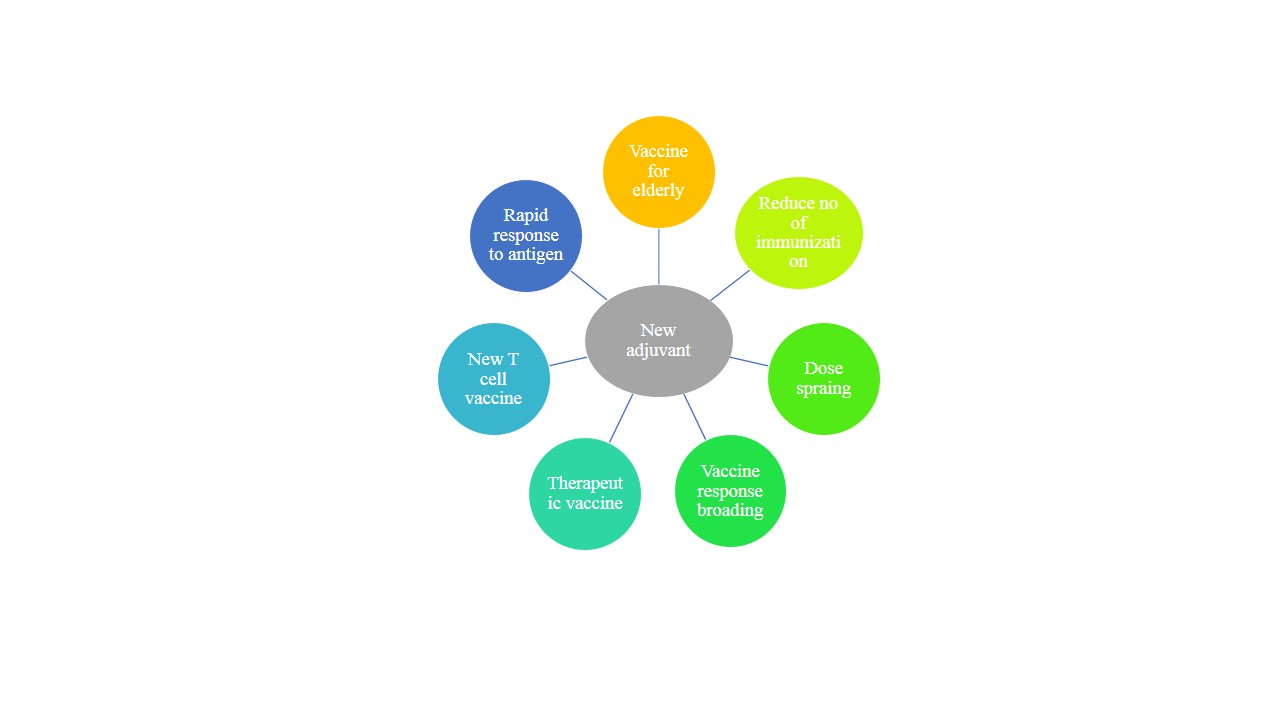
Fig no 3: benefits of adjuvant [48]
- Classification of adjuvant: [49]
Adjuvant are basically categorised based on their method of action, source, physiochemical properties and administration route.
- Mineral salt
- Tensoactive compound
- Microorganism derivative compound
- Emulsion
- Nanoparticles antigen delivery methods
- Liposome
- Polycrystalline microsphere
- Nano beds
- Virus like particle
- Cytokines polysaccharides
- Nuclei acid-based vaccine adjuvant
- Liposomes as vaccine adjuvant:
Out of all the other nanoparticle delivery techniques, liposomes, lipid vesicular structures, have shown the most promise as a vaccine delivery method [50]. The effective adjuvant and delivery technology known as liposomes is versatile and widely used. Despite the fact that most of their uses as agents for the administration of immunostimulatory molecules have been in the field of cancer immunotherapy, they can be thought of as a prime possibility for vaccine carriers. In addition to improving drug delivery, Employing liposomes as delivery methods and adjuvant for vaccines [51]. Targeted antigen distribution via a variety of administration routes is made possible by their versatility sizes, charges, and bilayer rigidity, and composition. Alec Bangham, a British haematologist, the first to discuss liposome technology [52]. He described liposomes as swollen phospholipid molecules with a complete variety in terms of size and structure systems for delivering drugs in small particles have the potential to serve as adjuvants [53]. To shield antigens from deterioration and to make antigen administration to antigen-presenting cells easier, they provide the capacity to insert subunit antigens within pathogen-sized particles [54]. Among the available technologies for delivering nanoparticle drugs, A result of its immunological function and adjuvant activity, the first system to be described was liposomes, Allison and Gregoriadis identified adjuvant characteristics (1974) [55]. The physicochemical properties of Adjuvants with Liposomes have a connection to the type of immunological response they promote, the biodistribution of liposomes, their exposure to lymph nodes, and the activation of the Innate defence mechanisms are all affected by the sizes and charge of the liposomes [56]. The encapsulated antigenic material is protected from the environment and released for an extessnded span of time. thanks to the liposomal adjuvant action, which improves Antigen-specific immune response and dendritic cell uptake. Inflexal (against influenza) and Epaxal (against hepatitis) These are the only two virosome-based vaccine systems that currently use liposomes as adjuvants [57].
| Adjuvant class | Response | Vaccine detailed | Ref |
| Cationic liposomes | 1]strong antigen-specific antibody and Th1/Th2 cell response 2] Immunostimulatory depot effect | vaccination against COVID-19 mRNA-1273, which is composed of lipid nanoparticles and nucleoside-modified mRNA.The SARS-CoV-2 spike is encoded (s)Glycoprotein
|
58 |
| Reformed liposomes | 1]strong antigen-specific antibody and Th1/Th2 cell response 2] Immunostimulatory depot effect | Tuberculosis Hybrid 1(H1) fusion protein combined with CAF01 liposome (NCT00922363)
component of the recombinant protein from Chlamydia trachomatis CTH 522 combined with CAF01 liposome (NCT02787109) |
59 |
Table no 3: types of liposomes adjuvant
A promising non-viral method of delivering human genes is cationic liposomes [60]. These liposomes typically consist of neutral phospholipids like dioleoylphosphatidyl ethanolamine and cationic lipid derivatives (DOPE). Widely Cationic liposome compositions include three different compound Transfectace, Transfectam, and DC-Cho 3BN-(N,N-dimethylaminoethane) carbamoyl-cholesterol. Cationic Dioleyloxypropyl Trimethylammonium Steroid liposomes (DOTMA) [61]. The liposomes’ physico-chemical characteristics have a major effect on how well they perform. The adjuvant impact is significantly influenced by the surface charge in particular, and Most in vivo investigations indicate that positive liposomes are preferable to normal and nonpolar liposomes [62]. The adjuvant effects of cationic liposomes are probably highly affected by their potential to choose antigens for APC endocytosis. According to Vangasseri et al, charged liposome made of ethylphosphocholine, such as 1.2-dioleoyl-sn-glycero-3-ethylphosphocholine (DOEPC), are more effective than those made of trimethylammonium propane lipids, like 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), at increasing DC maturation [63]. There is the wide range of cationic liposomes which are used as vaccine adjuvant.
- Adjuvant based on DOTPA: [64-67]
The quaternary ammonium compound DOTPA is positively charged and has two 18-carbon unsaturated hydrophobic fatty acid chains. Leventis and Silvius created it for the first time in 1990. Its structure consists of a 2-oleoyl chain-coated glycerol backbone joined to a quaternary amine head group. The difference between DOTMA and DOTAP is that ester bonds, not ether bonds, connect the chains to the backbone. A lipid’s sensitivity ranges from 25% to 30%. However, DOTAP is totally protonated at pH (7.4), necessitating the use of additional energy to successfully separate the DNA from the lipoplex. Therefore, DOTAP should be paired with a helper lipid, as seen in the majority of cationic lipid formulations, to be more successful in gene delivery. In this, lipoplex that exhibits resistance to serum interaction has been created using high temperature and a lengthy incubation period. At room temperature (T0°C), In aquatic conditions, the lipids self-organize into bilayers. The phase of these bilayers is fluid crystallization. According to Simberg et al. (1141), DOTAP-based liposomes are effective transfection agents, Additionally, it has been demonstrated that DOTAP liposomes improve immune responses to protein antigens 116–18 as well as DNA-encoded antigens. Clathrin is required for the endocytic pathway by which DOTAP mediates the endocytosis of antigens by APCs. According to certain research, DOTAP may also have immunomodulatory effects. Increased cytolytic T-cell activity after immunization using artificial enzymes or molecules demonstrates that how CD8 T-cell activation is brought on by DOTAP vesicle.
- Adjuvant based on DC-CHOL: [68-71]
It was first created in 1991 by “GAO and HUANG.” It has a cholesterol moiety that is joined to a hydrolysable dimethyl ethylene diamine by an ester link. Cholesterol is chosen because it contributes to lipid membranes and is biocompatible and stable. Similar to DOTAP, the cationic cholesterol derivative DC-Chol generates liposomes that significantly improve transfection and produce a balanced Th1/Th2 response in a variety of disease types. In addition, Guy et al. demonstrate that administration of DC-Chol lipid nanoparticles to mice by intranasal or subcutaneous injection can enhance antigen-specific antibody responses to the complexation of adult divided inhibited influenza vaccine. Furthermore, macaques produced substantial reactions against the DC-Chol adjuvanted vaccine that were antigen-specific, but the vaccine itself had little effect, not even after getting a supplemental vaccination. Additionally, it has been shown that DC-Chol liposomes improve the response of a plasmid DNA model vaccination an in-vitro investigation was conducted using epithelial cell lines that mimic the uterus and vagina.
- Adjuvant based on DDA lipid: [72-74]
Gall first discovered DDA as a potent adjuvant in 1966 after he showed that it could raise antibody titers against DT in guinea pigs. The quaternary ammonium DDA has a positive charge compound with two hydrophobic side chains that are 18-C long and saturated. DDA fatty acids organize themselves into liposomes in an aquatic environment. when heated over the altering stages point of their gel to liquid, which is 47°C.Hilgers and Snippe have evaluated the adjuvant qualities of DDA in great detail and think that DDA is safe due to trials showing that it has no negative effects on people. As per Larsen et al., Non-specific cell injury does not result in the adjuvant effect observed in mice, The study’s quaternary ammonium salts have an adjuvant effect without being toxic. The nostril epithelium is also in mice that had received DDA Using the nasal route and been inoculated with BBG2Na, an antigen for the respiratory virus, was unaffected.
| Lipid | Structure | Ref no |
|
DOTPA |
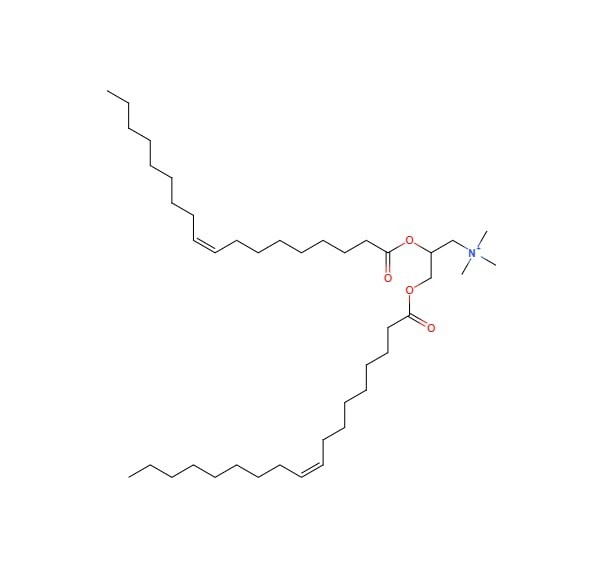 |
65 |
|
DC-Chol |
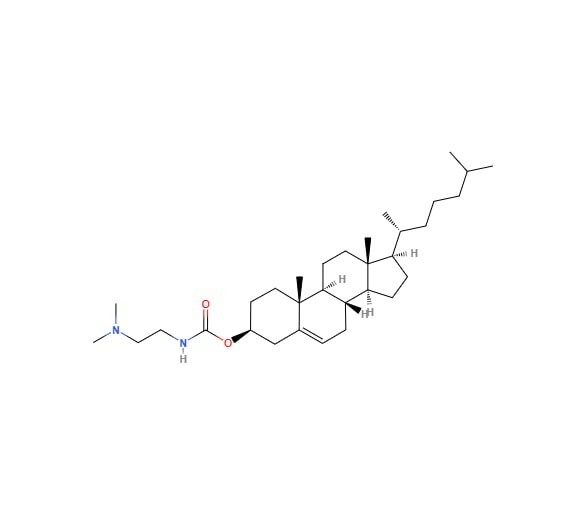 |
68 |
|
DDA |
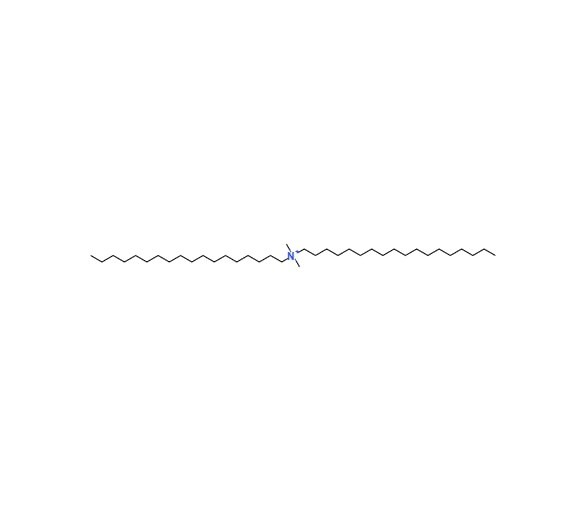 |
73 |
Table no 4: chemical structure of cationic liposomes
- Conclusion:
Liposomes have been applied in a variety of pharmaceutical techniques. The effectiveness of the liposomal composition relies on its ability to continually transport the therapeutic agent to the desired location while minimising its (the medication’s) side effects. Their versatility allows them to be applied to any pharmacological element and any mode of administration for the delivery of drugs, which independent of their characteristics. New vaccines are necessary to provide better healthcare throughout the world. The world’s population should have access to these vaccines, and they should be secure, efficient, and economical. Adjuvants for vaccines are essential for enhancing vaccination efficiency and durability. Because they are flexible and have a proven track record as delivery methods, liposomes provide an effective adjuvant platform.
- Reference
- Bangham AD. Development of the liposome concept. Liposomes in biological systems. 1980:1-24.
- Vishvakrama P, Sharma S. Liposomes: an overview. Journal of Drug Delivery and Therapeutics. 2014 Jun 24:47-55.
- Allen TM, Mehra T, Hansen C, Chin YC. Stealth liposomes: an improved sustained release system for 1-β-D-arabinofuranosylcytosine. Cancer Research. 1992 May 1;52(9):2431-9.
- Gregoriadis G, Florence AT. Liposomes in drug delivery. Drugs. 1993 Jan;45(1):15-28.
- Lim YT. Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease. Clinical and experimental vaccine research. 2015 Jan 1;4(1):54-8.
- Poolman JT. Shortcomings of pertussis vaccines: why we need a third generation vaccine. Expert review of vaccines. 2014 Oct 1;13(10):1159-62.
- Nisini R, Poerio N, Mariotti S, De Santis F, Fraziano M. The multirole of liposomes in therapy and prevention of infectious diseases. Frontiers in Immunology. 2018 Feb 5;9: 155.
- Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. npj Vaccines. 2019 Sep 24;4(1):1-1.
- Hope MJ, Bally MB, Mayer LD, Janoff AS, Cullis PR. Generation of multilamellar and unilamellar phospholipid vesicles. Chemistry and physics of lipids. 1986 Jun 1;40(2-4):89-107.
- Alving CR. Macrophages as targets for delivery of liposome-encapsulated antimicrobial agents. Advanced Drug Delivery Reviews. 1988 Oct 1;2(1):107-28.
- Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov;252(5480):252-.
- Deamer D, Uster P. Liposome preparation methods and monitoring liposome fusion. Introduction of Macromolecules into viable Mammalian Cells, Alan R. Liss, New York. 1980:205-20.
- De Marie S, Jankengt R, Bakker-Woudenberg IA. Clinical use of liposomal and lipid-complexed amphotericin B. Journal of Antimicrobial Chemotherapy. 1994 May 1;33(5):907-16.
- Lasic DD, Papahadjopoulos D. Liposomes revisited. Science. 1995 Mar 3;267(5202):1275-6.
- Emanuel N, Kedar E, Bolotin EM, Smorodinsky NI, Barenholz Y. Preparation and characterization of doxorubicin-loaded sterically stabilized immunoliposomes. Pharmaceutical research. 1996 Mar;13(3):352-9.
- Kunisawa J, Masuda T, Katayama K, Yoshikawa T, Tsutsumi Y, Akashi M, Mayumi T, Nakagawa S. Fusogenic liposome delivers encapsulated nanoparticles for cytosolic controlled gene release. Journal of controlled release. 2005 Jul 20;105(3):344-53.
- Wang CY, Huang L. Plasmid DNA adsorbed to pH-sensitive liposomes efficiently transforms the target cells. Biochemical and biophysical research communications. 1987 Sep 30;147(3):980-5.
- Shaheen SM, Shakil Ahmed FR, Hossen MN, Ahmed M, Amran MS, Ul-Islam MA. Liposome as a carrier for advanced drug delivery. Pak J Biol Sci. 2006;9(6):1181-91.
- Mishra H, Chauhan V, Kumar K, Teotia D. A comprehensive review on Liposomes: a novel drug delivery system. Journal of Drug Delivery and Therapeutics. 2018 Nov 25;8(6):400-4.
- Kataria S, Sandhu P, Bilandi AJ, Akanksha M, Kapoor B. Stealth liposomes: a review. International journal of research in ayurveda & pharmacy. 2011 Sep 1;2(5).
- Talsma H, Crommelin DJ. LIPOSOMES AS DRUG DELIVERY SYSTEMS. II: CHARACTERIZATION. Pharmaceutical technology. 1992;16(11):52-.
- Kegade P, Gade A, Sawant R, Parkar S. Liposomal drug delivery in Cancer. Asian Journal of Pharmaceutical Research. 2020 Nov 26;10(4):293-8.
- El Ridy MS, Khalil RM, Moustafa DM, El-Rashdy MS, Mohamed M, Osman A, Gaber MH, Monem AS. Chemoprophylaxis of schistosomiasis using liposome encapsulated oxamniquine. Drug development and industrial pharmacy. 1997 Jan 1;23(8):771-82.
- Deamer D, Bangham AD. Large volume liposomes by an ether vaporization method. Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis. 1976 Sep 7;443(3):629-34.
- Handa T, Sakano M, Naito S, Hiramatsu M, Tsuboi M. Thermal SiO and H13CO+ line observations of the dense molecular cloud G0. 11–0.11 in the Galactic Center Region. The Astrophysical Journal. 2006;636(1):261.
- Shaheen SM, Shakil Ahmed FR, Hossen MN, Ahmed M, Amran MS, Ul-Islam MA. Liposome as a carrier for advanced drug delivery. Pak J Biol Sci. 2006;9(6):1181-91.
- Meunier F, Prentice HG, Ringden O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. Journal of Antimicrobial Chemotherapy. 1991 Jan 1;28(suppl_B):83-91.
- Chou HH, Wang KL, Chen CA, Wei LH, Lai CH, Hsieh CY, Yang YC, Twu NF, Chang TC, Yen MS. Pegylated liposomal doxorubicin (Lipo-Dox®) for platinum-resistant or refractory epithelial ovarian carcinoma: A Taiwanese gynecologic oncology group study with long-term follow-up. Gynecologic oncology. 2006 Jun 1;101(3):423-8.
- Phuphanich S, Maria B, Braeckman R, Chamberlain M. A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt®) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. Journal of neuro-oncology. 2007 Jan;81(2):201-8.
- Leonard RC, Williams S, Tulpule A, Levine AM, Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet™). The Breast. 2009 Aug 1;18(4):218-24.
- Gardikis K, Tsimplouli C, Dimas K, Micha-Screttas M, Demetzos C. New chimeric advanced Drug Delivery nano Systems (chi-aDDnSs) as doxorubicin carriers. International journal of pharmaceutics. 2010 Dec 15;402(1-2):231-7.
- Hartrick CT, Hartrick KA. Extended-release epidural morphine (DepoDur™): Review and safety analysis. Expert review of neurotherapeutics. 2008 Nov 1;8(11):1641-8.
- Rodriguez MA, Pytlik R, Kozak T, Chhanabhai M, Gascoyne R, Lu B, Deitcher SR, Winter JN. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non‐Hodgkin lymphoma: report of the pivotal phase 2 study. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2009 Aug 1;115(15):3475-82.
- Participants VR. Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update. Retina. 2005 Feb 1;25(2):119-34.
- Chen E, Brown DM, Wong TP, Benz MS, Kegley E, Cox J, Fish RH, Kim RY. Lucentis® using Visudyne® study: Determining the threshold-dose fluence of verteporfin photodynamic therapy combined with intravitreal ranibizumab for exudative macular degeneration. Clinical Ophthalmology. 2010 Oct 5:1073-9.
- Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem. 2013 Mar;8(3):360-76.
- Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, Takahashi S, Tatem AJ, Wagner CE, Wang LF, Wesolowski A. Infectious disease in an era of global change. Nature Reviews Microbiology. 2022 Apr;20(4):193-205.
- Fan J, Jin S, Gilmartin L, Toth I, Hussein WM, Stephenson RJ. Advances in Infectious Disease Vaccine Adjuvants. Vaccines. 2022 Jul 13;10(7):1120.
- Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015 Apr 16;3(2):320-43.
- Vogel FR. Adjuvants in perspective. Developments in biological standardization. 1998 Jan 1;92: 241-8.
- Nikam NR, Patil PR, Vakhariya RR, Magdum CS. Liposomes: A Novel Drug Delivery System: An Overview. Asian J. Pharm. Res. 2020 Mar 9;10(1):23-8.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nature medicine. 2013 Dec;19(12):1597-608.
- Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A (H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. Journal of autoimmunity. 2014 May 1;50: 1-11.
- Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug safety. 2015 Nov;38(11):1059-74.
- Mitkus RJ, King DB, Hess MA, Forshee RA, Walderhaug MO. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine. 2011 Nov 28;29(51):9538-43.
- RTS, S Clinical Trials Partnership. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. New England Journal of Medicine. 2011 Nov 17;365(20):1863-75.
- Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011 Nov 28;29(51):9563-72.
- Andersson LC, Häyry P, Bach MA, Bach JF. Differences in the effects of adult thymectomy on T-cell mediated responses in vitro. Nature. 1974 Nov;252(5480):252-4.
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet. 2012 Feb 4;379(9814):413-31.
- Lim YT. Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease. Clinical and experimental vaccine research. 2015 Jan 1;4(1):54-8.
- Taneichi M, Ishida H, Kajino K, Ogasawara K, Tanaka Y, Kasai M, Mori M, Nishida M, Yamamura H, Mizuguchi J, Uchida T. Antigen chemically coupled to the surface of liposomes are cross-presented to CD8+ T cells and induce potent antitumor immunity. the Journal of Immunology. 2006 Aug 15;177(4):2324-30.
- Conacher M, Alexander J, Brewer JM. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine. 2001 Apr 6;19(20-22):2965-74.
- Calcagnile S, Zuccotti GV. The virosomal adjuvanted influenza vaccine. Expert opinion on biological therapy. 2010 Feb 1;10(2):191-200.
- Baillie AJ, Florence AT, Hume LR, Muirhead GT, Rogerson A. The preparation and properties of niosomes—non‐ionic surfactant vesicles. Journal of pharmacy and pharmacology. 1985 Dec;37(12):863-8.
- Almeida J, Edwards DC, Brand C, Heath T. Formation of virosomes from influenza subunits and liposomes. The Lancet. 1975 Nov 8;306(7941):899-901.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ. An mRNA vaccine against SARS-CoV-2—preliminary report. New England journal of medicine. 2020 Jul 14.
- van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA, O’Dee DM, Graves A, Thierry-Carstensen B, Andreasen LV, Ruhwald M. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014 Dec 12;32(52):7098-107.
- Abraham S, Juel HB, Bang P, Cheeseman HM, Dohn RB, Cole T, Kristiansen MP, Korsholm KS, Lewis D, Olsen AW, McFarlane LR. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. The Lancet infectious diseases. 2019 Oct 1;19(10):1091-100.
- Goyal P, Goyal K, Kumar SV, Singh AO, Katare OP, Mishra DN. Liposomal drug delivery systems–clinical applications. Acta Pharm. 2005 Mar 1;55(1):1-25.
- Kim A, Lee EH, Choi SH, Kim CK. In vitro and in vivo transfection efficiency of a novel ultradeformable cationic liposome. Biomaterials. 2004 Jan 1;25(2):305-13.
- Yoshida J, Mizuno M, Fujii M, Kajita Y, Nakahara N, Hatano M, Saito R, Nobayashi M, Wakabayashi T. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon β gene using cationic liposomes. Human gene therapy. 2004 Jan 1;15(1):77-86.
- Shi Y, Rock KL. Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. European journal of immunology. 2002 Jan;32(1):155-62.
- Simberg D, Weisman S, Talmon Y, Barenholz Y. DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2004;21(4).
- Perrie Y, McNeil S, Vangala A. Liposome-mediated DNA immunisation via the subcutaneous route. Journal of drug targeting. 2003 Jan 1;11(8-10):555-63.
- Vangala A, Bramwell VW, McNeil S, Christensen D, Agger EM, Perrie Y. Comparison of vesicle based antigen delivery systems for delivery of hepatitis B surface antigen. Journal of controlled release. 2007 May 14;119(1):102-10.
- Zubom IS Kalicharan R. Hoekstra D Lipples dated transfection of mammalian cris series through the thelested dependret clathris media puthway of endocytais Ch 2770201 18021-18028 (2002).
- Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochemical and biophysical research communications. 1991 Aug 30;179(1):280-5.
- Brunel F, Darbouret A, Ronco J. Cationic lipid DC-Chol induces an improved and balanced immunity able to overcome the unresponsiveness to the hepatitis B vaccine. Vaccine. 1999 Apr 23;17(17):2192-203.
- Sanchez V, Gimenez S, Haensler J, Geoffroy C, Rokbi B, Seguin D, Lissolo L, Harris B, Rizvi F, Kleanthous H, Monath T. Formulations of single or multiple H. pylori antigens with DC Chol adjuvant induce protection by the systemic route in mice Optimal prophylactic combinations are different from therapeutic ones. FEMS Immunology & Medical Microbiology. 2001 Mar 1;30(2):157-65.
- Guy B, Pascal N, Françon A, Bonnin A, Gimenez S, Lafay-Vialon E, Trannoy E, Haensler J. Design, characterization and preclinical efficacy of a cationic lipid adjuvant for influenza split vaccine. Vaccine. 2001 Feb 8;19(13-14):1794-805.
- Cremel M, Hamzeh-Cognasse H, Genin C, Delézay O. Female genital tract immunization: evaluation of candidate immunoadjuvants on epithelial cell secretion of CCL20 and dendritic/Langerhans cell maturation. Vaccine. 2006 Jul 17;24(29-30):5744-54.
- Katz D, Lehrer S, Galan O, Lachmi B, Cohen S, Inbar I, Samina I, Peleg B, Heller D, Yadin H, Chai D. Unique immunomodulating properties of dimethyl dioctadecyl ammonium bromide (DDA) in experimental viral vaccines. Novel strategies in the design and production of vaccines. 1996:115-25.
- Klinguer-Hamour C, Libon C, Plotnicky-Gilquin H, Bussat MC, Revy L, Nguyen T, Bonnefoy JY, Corvaı̈a N, Beck A. DDA adjuvant induces a mixed Th1/Th2 immune response when associated with BBG2Na, a respiratory syncytial virus potential vaccine. Vaccine. 2002 Jun 21;20(21-22):2743-51.
- Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infection and immunity. 1997 Feb;65(2):623-9.
