|
Smita Borkar*, Dr. Hemant Yadav, Dr. Abhay Raizaday School of Pharmacy, Suresh Gyan Vihar University, Jaipur, India Arvind Gavali college of Pharmacy, Jaitapur, Satara, Maharashtra, India |
| Abstract:
Skin injury is one of the most common lesions that people have to face, and some wounds, such as infected wounds and extensive burns, are notably difficult to heal. Nano formulations based wound healing has tremendous potential for treating and preventing wound infections with its multiple benefits compared with traditional treatment approaches. Nanotechnology presents a once-in-a-lifetime potential to revolutionize and invent new cures, as well as improve the efficacy of existing medical treatments. Nano-drug delivery methods, in particular, anchor bioactive molecules to the treated area, maintain drug release, and expressly improve drug therapeutic efficacies, making such a fine figure in the field of skin regeneration. This creates awareness regarding and addressed the existing nano-drug delivery systems with tremendous possibilities for wound healing and skin regeneration, with a focusing on liposomes, polymeric nanoparticles, inorganic nanoparticles, lipid nanoparticles, nanofibrous and nanohydrogel.
Keywords Nano formulations, wound healing, nanofibers, nanoparticles. |
Introduction:
Wounds: Wounds result from disruption of the normal anatomical epithelial lined tissue barriers and may be caused by trauma, tissue resection, or burns [1]. Some wounds are failed to heal in a less time and become chronic. This occurs in conditions such as diabetes or peripheral vascular disease. Failure to heal might also result from post-operative wound infections which are estimated to affect up to 4% of patients who undergo surgery. Chronic non-healing wounds represent a growing health and economic burden and are associated with a high morbidity that adds significantly to the cost of medical care [2]. The gold standard for the treatment of non-healing skin wounds is the transplantation of autologous skin. Clearly, there is a need to develop strategies to promote wound healing and prevent scarring [3,4].
Types of Wounds: Wounds are classified into two categories: acute and chronic wounds [5] depending on the methods and duration of the healing process.
Acute wounds: Wounds resulting from corrosive chemicals, radioactivity, mechanical injury, heat, or electrical shock, they usually heal with proper wound-care treatment in a fairly short period of time. Chronic wounds are associated with specific diseases like diabetes mellitus, and do not follow the orderly set of stages and predictable amount of time that characterize the normal wound-healing process. Chronic wounds frequently remain in the inflammatory stage for a long time, and their duration is associated with factors like bacterial load, necrotic tissue, and moisture balance of the wound site. Later, the risks of this regeneration of chronic wounds are high, unless the root of the disease is cured [6]. Sometimes chronic wounds can never heal or it may take many years to heal.
Wounds classification according to wound depth:
- Superficial wounds, which lost a part of epidermis
- Partial thickness wounds, here deeper dermal and epidermis layer are affected
- Full thickness wounds, here the deeper tissue and subcutaneous fat are ruptured [7,8].
Wound healing: Wound healing is a dynamic response to injury. In addition, mast cells and macrophages play a crucial role in repairing wounds [9]. Wound healing is divided into three stages which overlap in time (figure) – haemostasis and inflammation; proliferation; and remodelling and maturation. Irrespective of the cause of their infliction, all wounds go through these three phases [10].
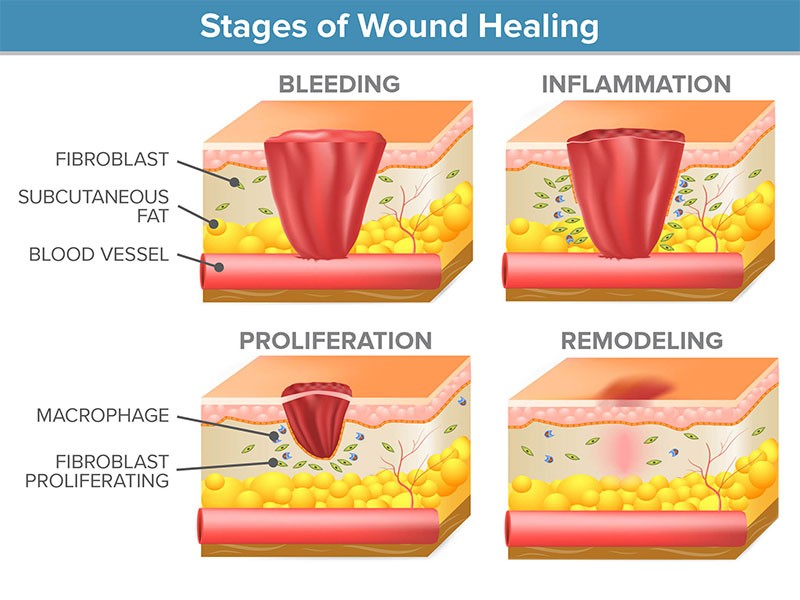
Figure: stages of wound healing
Haemostasis occurs just after the injury it utilizes clotting factors which prevent further blood loss from the wound site and structural foundation for the future formation of granulation tissue. The inflammation phase involves phagocytic cells that release reactive oxygen species, it may last for up to seven days in acute wounds and longer in chronic wounds.
As inflammatory cells undergo apoptosis and wound healing progresses to the phase of proliferation, that is characterized by the formation of granulation tissue, wound contraction, angiogenesis (blood vessel formation) and the epithelialization process. The remodelling phase, that is characterized by the formation of scar tissue and it may occur over a period of months and or years, depending on the initial severity of the wound, method of treatment and location. [11].
Nanotechnology: By downscaling to the nanometric range, a particle’s surface increases exponentially, whereas its volume decreases, which leads to particular physicochemical characteristics that account for numerous medical applications. The shape and size of nanoparticles are important properties that determine their biological efficiency by influencing active substance delivery, penetrability and cellular responses.
Nano‑drug delivery system in wound treatment and skin regeneration Nano-DDSs hold immense potential in enhancement of drug therapeutic efficacy for their capability of preventing drug degradation and sustaining drug release. Nano-DDSs including lipid nanoparticles, liposomes, nanohydrogel, polymeric nanoparticles, inorganic nanoparticles, nanofibrous structures [12-14].
Nano-DDSs clearly speed wound healing and increase healing quality due to the numerous benefits they provide [4,5]:
- Nano-DDSs have been discovered to be non-toxic, skin-compatible, and to facilitate wound healing activation and acceleration by offering a moist environment.
- Some nano-DDSs have the ability to overcome cellular barriers and reach the cytoplasmic space, as well as activate specific transport systems to promote drug retention.
- When combined with bioactive molecules, nano-DDSs shield pharmaceuticals from protease-mediated degradation in wounds, significantly improving therapeutic efficacy.
- Sustained drug release minimizes the frequency of administration, lowers expenditure, and appropriately address while extending the maintenance of an active therapeutic content. In this systematic review, nanotechnologies are examined with reference to wound healing.
Recent researches of nano-DDSs are listed in following table
Table: Recent research of nano-drug delivery system in wound treatment and skin regeneration
| Formulation | Drug | Administration | Outcome |
| Deformable liposomes | Curcumin | Topical treatment, once a day for 18 days | Shorten inflammatory process, prevent infection and promoted fibrosis, angiogenesis, re-epithelialization and wound contraction [49]. |
| Nanoparticles | Thrombin | Topical treatment | Advanced process of healing, improved skin tensile strength, reduced complications in surgery [28]. |
| Hydrogel loading nanoparticles
|
Asiatic acid/ZnO/CuO
|
Topical treatment
|
Raised DNA, total protein, hexosamine and
hydroxyproline content and rendered superior re-epithelization, collagen fibres arrangement and angiogenesis [53]. |
| Microspheres/scaffold
|
Mupirocin | Applied topically, covered and tied | Combated infection and stimulated fibroblast proliferation and dermal collagenization [54]. |
| SLNs
|
LL37 and A1
|
Applied topically
|
Promoted wound closure in fibroblast cells and keratinocytes, simultaneously enhanced antibacterial activity [40]. |
| Nanofibers loaded with nanoparticles
|
Cefazolin/zinc oxide
|
Applied topically/single
|
Showed great anti-bacterial activity, enhanced cell adhesion and epithelial migration, contributed to faster and more efficient collagen synthesis [56]. |
| Nanofibrous scaffold
|
Human bone marrow stem cells | Applied topically
|
Boosted cell growth rate and accelerated wound Recovery [56].
|
| Hybrid nanostructures/hydrogel | Ag/Ag–AgCl/ZnO
|
Applied every 2 days for 14 days | Stimulated the immune function, produced the synergistic antibacterial effects and accelerated wound healing. |
| Nano
hydrogel
|
Baicalin
|
Topically smeared every day for 4 days | Faster and more complete skin restoration and inhibition of specific inflammatory markers was noticed [52]. |
Liposomes: Liposomes are bilayer vesicles built by amphiphilic molecules such as phospholipids, emerging as one of promising nano-carriers for topical drug delivery [15]. They are nontoxic, biodegradable, biocompatible with skin, and capable of accommodating both hydrophilic drugs (example: growth factors) in inner water cavity and hydrophobic agent in bilayer [16, 17]. Furthermore, liposomes effectively cover wound and create moist environment on wound surface after application, which is very conducive to wound healing [18]. All these merits of liposomes have been universally applied in wound treatment and skin regeneration. Xu et al. [19] prepared a novel liposome with hydrogel core of silk fibroin which effectively encapsulated bFGF. The vehicles improved the stability of bFGF in wound fluids and maintained cell proliferation activity with respect to traditional liposomes.
Polymeric nanoparticles: Polymeric nanoparticles are biocompatible colloidal systems drawing increasing attention in both biomedicine and bioengineering fields [20]. When drugs are embedded with these polymeric devices, drugs get protected from degradation by the proteases presenting in the wound and released in a controlled manner so as to reduce administration frequency. The need of effectively delivering biomolecules such as antimicrobial agents, growth factors and genes, will be met with aid of nanoparticles [21, 22]. Currently most polymeric nanoparticles are prepared by poly lactic-co-glycolic acid (PLGA, crowned as the mostly used polymers), alginate, gelatine, chitosan, as well as other polymer combinations [23, 24]. Dave et al. prepared a lipid polymer hybrid nanoparticle formulation which was able to sustained drug release to 24 h with favourable skin permeation and reduced the frequency of application [25].
To reduce more cytotoxicity of Amphotericin B, Sanchez et al. incorporated Amphotericin B into the silane- based hydrogel nanoparticles to replace the intravenous injection infusion (traditional). Amphotericin B nanoparticles resulted in equivalent or enhanced killing efficacy with 72.4–91.1% by 4 h for Clinical strains [26].
Inorganic nanoparticles: Inorganic nanoparticles refer to nanoparticles deprived from inorganic materials, including the metallic nanoparticles, carbon-based nanoparticles, ceramic nanoparticles etc. [27]. Inorganic nanoparticles exhibit similar merits in wound healing treatment and strong antibacterial effect. Ali et al. designed and synthesized ZnO2 nanoparticles by co-precipitation method. Zinc oxide nanoparticles had good antibacterial activity for aspergillus and pseudomonas aeruginosa isolated from wound infected tissues of burn patients. Due to their antibacterial properties and low toxicity profile, metal nanoparticles such as silver, gold and zinc represent ideal candidates for integration in wound dressings [28].
- Silver Nanoparticles
Silver nanoparticles (AgNPs) is the metallic nanoparticle it overcome the limitations of standard silver compounds. Due to their high surface volume ratio, AgNPs are more potent at less concentration, thus lowering their toxicity. Pure AgNPs can modulate anti-inflammatory cytokine release in order to promote rapid wound closure without increasing scarring. By inducing the differentiation of myofibroblasts from normal fibroblasts, Silver Nanoparticles promote wound contractility thus accelerating the healing process. Moreover, AgNPs stimulate epidermal re-epithelialization through the proliferation and relocation of keratinocytes [31]. However, Szmyd et al. reported that high concentration of AgNPs decrease keratinocyte viability, migration, metabolism and differentiation of these cells, through the activation of caspase 7 and 3 and dose-dependent DNA damage [32]. For side effects reduction, silver nanoparticles can be used in low doses along with the antimicrobial drugs to achieve high efficiency. In a recent study it was demonstrated that AgNPs combined with tetracycline significantly decreased bacterial load both in superficial and deep tissue layers in a mouse model, thus accelerating healing [33]. This nanomaterial proved a high bacteria-killing performance against Gram negative pathogens and promoted wound healing [34, 35].
- Gold Nanoparticles
Gold nanoparticles (AuNPs) represent a option when it comes to wound therapy due to their capacity of absorbing near infrared light, while, at the same time and chemical stability, being relatively easy to synthetize [30]. Gold Nanoparticles can either directly target the bacterial cell wall, or they can bind to bacterial DNA, blocking the double-helix from uncoiling during replication or transcription, thus exerting bactericidal and bacteriostatic properties. As a result, they can inhibit multidrug resistant pathogens like Pseudomonas aeruginosa and Staphylococcus aureus. Moreover, AuNPs prevent the formation of reactive oxygen species, thus acting as antioxidants, aiding the healing process [31].
- Zinc Oxide Nanoparticles
Zinc oxide nanoparticles (ZnONPs) itself represent a reliable antibacterial agent by inducing the bacterial cell membrane perforations. Moreover, when incorporated in hydrogel-based wound dressings, the overall contact time is increased, promoting keratinocyte migration, thus improving re-epithelialization [31]. In addition, in a recent study, a microporous chitosan hydrogel/ZnONPs dressing presented a high capacity of absorbing wound exudates and enabled the formation of haemostatic blood clots, while also displaying antibacterial properties with little cytotoxicity [29].
Lipid nanoparticles: Nanostructured lipid carriers (NLCs) and Solid lipid nanoparticles (SLNs) are the lipid nanoparticles to overcome the limitation of liposomes. Lipid nanoparticles were generally prepared with physiological lipids or lipid molecules and their preparation process requires no involvement of any potentially toxic organic solvents [36-40].
Nanofibrous structures: Nanofibers are fabricated from natural and synthetic polymer chains which are able to act as nanofibrous sheets or 3D-scafolds applied in the tissue engineering [41, 42]. These nanofibrous structures are designed to mimic the ECM, provide favourable condition for cell attachment and elevate cell-drug interaction, serving as a replacement for artificial dermal analogues [42, 43]. Electrospinning is mostly used technique for production of nanofibers. An electrical charge is taken as driving force to draw fibres from a polymer solution so as to fabricate nanometric continuous fibres [44]. Due to its high area and volume ratio the nanofibers enhance transfer of the variety of therapeutic agents include diverse antimicrobial agents, nucleic acids, growth factor [45,47,57].
Nanohydrogel: Nanohydrogel is the three dimensional polymeric networks considered as best formulation for the wound treatment, the porous three dimensional structure enrich it with the ability of preventing wound dehydration, aqueous fluid absorption [48] and creating a beneficial moist environment for wound healing [49], its non-adhesive nature allows it to preserve the wound bed while maintaining the penetration of oxygen, which is necessary for the wound healing [50]; Texture (soft) of nanohydrogel provides comfortable experience in the course of treatment [51]. Nanohydrogel is able to encapsulate many drugs with efficacy and perfect compatibility exerting an impressive effect on skin regeneration [52].
Conclusions and Future Perspectives:
The main purpose of this review was to highlight the advantages of nano formulations for the wound-healing process. The wound-healing process based on nanomaterial also proved to be more effective than the conventional wound therapy. Nanomaterials alter wound healing phases of the wound healing process since they possess anti-proliferative, antibacterial, anti-inflammatory properties.
Wound healing is an intricate three-staged process involving inflammation, proliferation and remodelling. However, chronic wounds still remain a challenge due to multidrug-resistant microorganisms, bacteria biofilms and because of current therapies mostly failed to provide favourable outcomes of wound healing, due to the Superior surface to volume ratio of nanoparticle can be efficiently employed in medical applications including wound therapy. Inorganic nanoparticles such as silver, gold and zinc possess good properties like antibacterial activity and low toxicity for integration in wound dressings. Moreover, nano compounds which encapsulate growth factors, genes or stem cells are currently under development, thus offering new treatment modalities in the near future. The nano-DDSs in recent years has brought new insight for skin regeneration of wounds, benefits like drug carriers prolong drug release, protect drug from degradation and improve skin retention, so as to realize augment of the therapeutic power of biological and synthetic molecules (example: reduction or eradication of the wound bacterial load and improvement of re-epithelialization).
In current times, huge progress has been made in discovering the mechanisms underlying the wound healing process. In current clinical treatments of wounds, medications such as topical antimicrobial agents are still relevant. Moreover, applying nanotechnology and incorporating knowledge of cellular, subcellular events occurring during the typical healing process, could obviously get better future therapeutic interventions. Nanotechnology offers high opportunities for improving wound treatments. The nanometre scale opens the way for the development of novel materials for use in highly advanced medical technology.
Reference:
- Diegelmann RF. Cellular and biochemical aspects of normal and abnormal wound healing: an overview. J Urol 157, 298-302 (1997) DOI: 10.1016/S0022-5347(01)65364-3
- Metcalfe AD, Ferguson MWJ. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 4, 413-37 (2007) DOI: 10.1098/rsif.2006.0179
- Atala A. et. al., Wound Healing Versus Regeneration: Role of the Tissue Environment in Regenerative Medicine. MRS Bull 35, (2010) DOI: 10.1557/mrs2010.528
- Naghmeh Naderi et.al, Nanoparticles in wound healing; from hope to promise, from promise to routine Article in Frontiers in Bioscience · January 2018
- Wang, W. et. al., Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotechnol. 2019, 17, 82.
- Upton, D.; Solowiej, K.; Hender, C.; Woo, K. Stress and pain associated with dressing change in patients with chronic wounds. J. Wound Care 2012, 21, 53–61.
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267.
- Review Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics Atanu Naskar and Kwang-sun Kim
- Young A, McNaught CE. The physiology of wound healing. Surgery 2011;29(10):475-479. doi: 10.1016/j.mpsur.2011.06.011
- Gaje PN. et al. Mast cells: key players in the shadow in oral inflammation and in squamous cell carcinoma of the oral cavity [Internet]. BioMed Res Int 2016. doi: 10.1155/2016/9235080
- de Oliveira Gonzalez AC. Et. al., Wound healing: a literature review.
- An Bras Dermatol 2016 Sep–Oct;91(5):614–620. doi: 10.1590/ abd1806-4841.20164741.
- Gainza G, Villullas S. et. al., Advances in drug delivery systems (DDSs) to release growth factors for wound heal‑ ing and skin regeneration. Nanomedicine. 2015;11:1551–73.
- Navarro M, Planell JA. Nanotechnology in regenerative medicine. Drug Deliv Syst. 2011;21:623–6. 14. Korrapati PS, Karthikeyan K, Satish A, Krishnaswamy VR, Venugopal JR, Ramakrishna S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater Sci Eng C Mater Biol Appl. 2016;67:747–65.
- Chen J et al, Infu‑ ence of lipid composition on the phase transition temperature of liposomes composed of DPPC and HSPC. Drug Dev Ind Pharm. 2013;39:197–204.
- Mitragotri S. et. al, Overcoming the challenges in adminis‑ tering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–72.
- Degim Z, Celebi N, Alemdaroglu C, Deveci M, Ozturk S, Ozogul C. Evalu‑ ation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. Int Wound J. 2011;8:343–54.
- Manca ML, Matricardi P, Cencetti C, Peris JE, Melis V, Carbone C, Escribano E, Zaru M, Fadda AM, Manconi M. Combination of argan oil and phospholipids for the development of an efective liposome-like formulation able to improve skin hydration and allantoin dermal deliv‑ ery. Int J Pharm. 2016;505:204–11.
- Xu HL, Chen PP, ZhuGe DL, Zhu QY, Jin BH, Shen BX, Xiao J, Zhao YZ. Liposomes with silk fbroin hydrogel core to stabilize bFGF and pro‑ mote the wound healing of mice with deep second-degree scald. Adv Healthc Mater. 2017;6:1700344.
- Huang S, Fu X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J Control Release. 2010;142:149–59.
- Yun YH, Goetz DJ, Yellen P, Chen W. Hyaluronan microspheres for sustained gene delivery and site-specifc targeting. Biomaterials. 2004;25:147–57.
- Chu Y, Yu D, Wang P, Xu J, Li D, Ding M. Nanotechnology promotes the full-thickness diabetic wound healing efect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010;18:499–505.
- Ye M, Kim S, Park K. Issues in long-term protein delivery using biode‑ gradable microparticles. J Control Release. 2010;146:241–60.
- Gainza G, Aguirre JJ, Pedraz JL, Hernández RM, Igartua M. rhEGFloaded PLGA-Alginate microspheres enhance the healing of fullthickness excisional wounds in diabetised Wistar rats. Eur J Pharm Sci. 2013;50:243–52
- Dave V et. Al, Hybrid nanoparticles for the topical delivery of norfoxacin for the efective treatment of bacterial infection produced after burn. J Microencapsul. 2017;34:351–65.
- Sanchez DA et. Al, Amphotericin B releasing nanoparticle topical treatment of Candida spp. in the set‑ ting of a burn wound. Nanomedicine. 2014;10:269–77.
- Mofazzal Jahromi MA et. Al, Nanomedicine and advanced technologies for burns: prevent‑ ing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64.
- Ali SS et. Al, Synthesized zinc peroxide nanoparticles (ZnO2-NPs): a novel antimicrobial, antielastase, anti-keratinase, and anti-infammatory approach toward polymicrobial burn wounds. Int J Nanomed. 2017;12:6059–73.
- Kumar P.T. Al, Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces. 2012;4:2618–2629. doi: 10.1021/am300292v.
- Niska K. Al, Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chem. Biol. Interact. 2018;295:38–51. doi: 10.1016/j.cbi.2017.06.018.
- Vijayakumar V. Al, Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019;122:137–148. doi: 10.1016/j.ijbiomac.2018.10.120.
- Szmyd R., Drukala J., et al. Effect of silver nanoparticles on human primary keratinocytes. Chem. 2013;394:113–123. doi: 10.1515/hsz-2012-0202.
- Ahmadi M., Adibhesami M. The Effect of Silver Nanoparticles on Wounds Contaminated with Pseudomonas aeruginosa in Mice: An Experimental Study. J. Pharm. Res. 2017;16:661–669.
- Pal S., Nisi R., Stoppa M., Licciulli A. Silver-Functionalized Bacterial Cellulose as Antibacterial Membrane for Wound-Healing Applications. ACS Omega. 2017;2:3632–3639. doi: 10.1021/acsomega.7b00442.
- Radulescu M. et al. Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings. 2016;9:345. doi: 10.3390/ma9050345.
- Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery sys‑ tem for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–90.
- Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribu‑ tion and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107:215–28.
- Silva AC et. Al, Solid lipid nanoparticles (SLN)-based hydrogels as potential carriers for oral transmucosal delivery of risperidone: preparation and characteriza‑ tion studies. Colloids Surf B. 2012;93:241–8.
- Souto EB, Müller RH. SLN and NLC for topical delivery of ketocona‑ zole. J Microencapsul. 2005;22:501–10.
- Gainza G et. Al, novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparti‑ cles: in vitro bioactivity and in vivo efectiveness in healing-impaired db/db mice. J Control Release. 2014;185:51–61.
- Hromadka M et. Al, Nanofber applications for burn care. J Burn Care Res. 2008;29:695.
- Reddy VJ et. Al, Nanofbrous structured biomi‑ metic strategies for skin tissue regeneration. Wound Repair Regen. 2013;21:1–16.
- Tocco I, Zavan B, Bassetto F, Vindigni V. Nanotechnology-based thera‑ pies for skin wound regeneration. J Nanomater. 2012;2012:4.
- Yang Y et. Al, Promotion of skin regeneration in diabetic rats by electrospun core-sheath fbers loaded with basic fbroblast growth factor. Biomaterials. 2011;32:4243–54.
- Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of poly‑ meric nanofbers for drug delivery applications. J Control Release. 2014;185:12–21.
- Zulkifi FH, A facile synthesis method of hydroxyethyl cellulose-silver nanoparticle scafolds for skin tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2017;79:151–60.
- Fan X, Chen K, He X, Na L, Huang J, Tang K, Li Y, Fang W. Nano-TiO2/colla‑ gen-chitosan porous scafold for wound repairing. Int J Biol Macromol. 2016;91:15–22
- Bhattacharya M, Malinen MM, Lauren P, Lou YR, Kuisma SW, Kanninen L, Lille M, Corlu A, Guguen-Guillouzo C, Ikkala O. Nanofbrillar cellulose hydrogel promotes three-dimensional liver cell culture. J Control Release. 2012;164:291–8.
- Pachuau L. Recent developments in novel drug delivery systems for wound healing. Expert Opin Drug Deliv. 2015;12:1895–909.
- Anumolu SS, et. Al, Doxycycline hydrogels with reversible disulfde crosslinks for dermal wound healing of mustard injuries. Biomaterials. 2011;32:1204–17.
- Hajimiri M et. Al, Preparation of hydrogel embedded polymer-growth factor conjugated nanoparticles as a diabetic wound dressing. Drug Dev Ind Pharm. 2015;42:1.
- Manconi M et. Al, Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur J Pharm Biopharm. 2018;127:244–9
- Thanusha AV, Dinda AK, Koul V. Evaluation of nano hydrogel compos‑ ite based on gelatin/HA/CS sufused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater Sci Eng C Mater Biol Appl. 2018;89:378–86.
- Kim MH et. Al, Injectable methyl‑ cellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr Polym. 2018;181:579–86.
- Lokhande G et. Al, Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018;70:35–47
- Qiao‑ling Huang1 et. Al, Nano-drug delivery systems in wound treatment and skin regeneration, 2019.
- Borkar, S. P., & Raizaday, A. (2022). Electrospun nanofibers a novel treatment for localized applications: A review. International Journal of Health Sciences, 6(S3), 12336– 12347. https://doi.org/10.53730/ijhs.v6nS3.9061
