Research Paper
Vol.7 Issue 2 Page No 21-23
¹ Neetu Sharma Gaur, ² Dr. R.C Chhipa
¹ Research Scholar, Department of Chemistry, Suresh Gyan Vihar University, Jaipur
2 Professor, Department of Chemistry, Suresh Gyan Vihar University, Jaipur
neetugaur88@gmail.com, rc.chhipa@mygyanvihar.com
Corresponding author: Neetu Sharma Gaur, Department of Chemistry, Suresh Gyan Vihar University, Jaipur
E-mail address: neetugaur88@gmail.com
Abstract– Carbon nanoparticles less then 10 nm. Which are generally small size carbon quantum dots.carbon quantum dots with special properties have generally become a new nano carbon member.In this paper we have prepared carbon quantum dots by hydrothermal method using the extract of aamla fruit used as a carbon saurce andliquid ammonia as a solvent.The carbon quantum dots which is prepared by hydrothermal method is analysed by UV and IR spectroscopy.
I INTRODUCTION
Amla is the most extensively studied plant.Reports suggested that it contain tannins alkaloid sand phenols. Fruit have 28% of the total tannins distributed in the whole plant. Fruit contain two hydrolysable tannins Emblicanin A and B. which have antioxidant properties. The amla fruit is quite fibrous and sour and bitter taste.The fruit contain phyllemblin activity directed fractionation revealed the presence of sevral phytochemicals like quercetin alkaloids like phyllantin and phyllantidine are found.In India amla is commonly used as adiuretic, liver tonic, refrigerant an antipyretic hair tonic, an ulcer preventive and for treatment of common cold.

Figure-1 Phyllanthus Emblica (a) A hurb

Figure-1 Phyllanthus Emblica (b) Powderd
Amla extract is used as a carbon saurce and ammonia solution. Without adding any surface passivating agent hydrothermal carbonization takes place. Ammonia was added in acidic medium to improve the photophysical property of CQDs.
Experimental Method:- Materials and Method –
- emblica (amla) powder – 5 gm
- Aqcous Ammonia – 30 ,Concn H2SO4, Conc HNO3
- Distilled water
Test tibes, Beakes, fumel, boiling tubes test tube stand, watch glass, filter paper etc
II METHOD
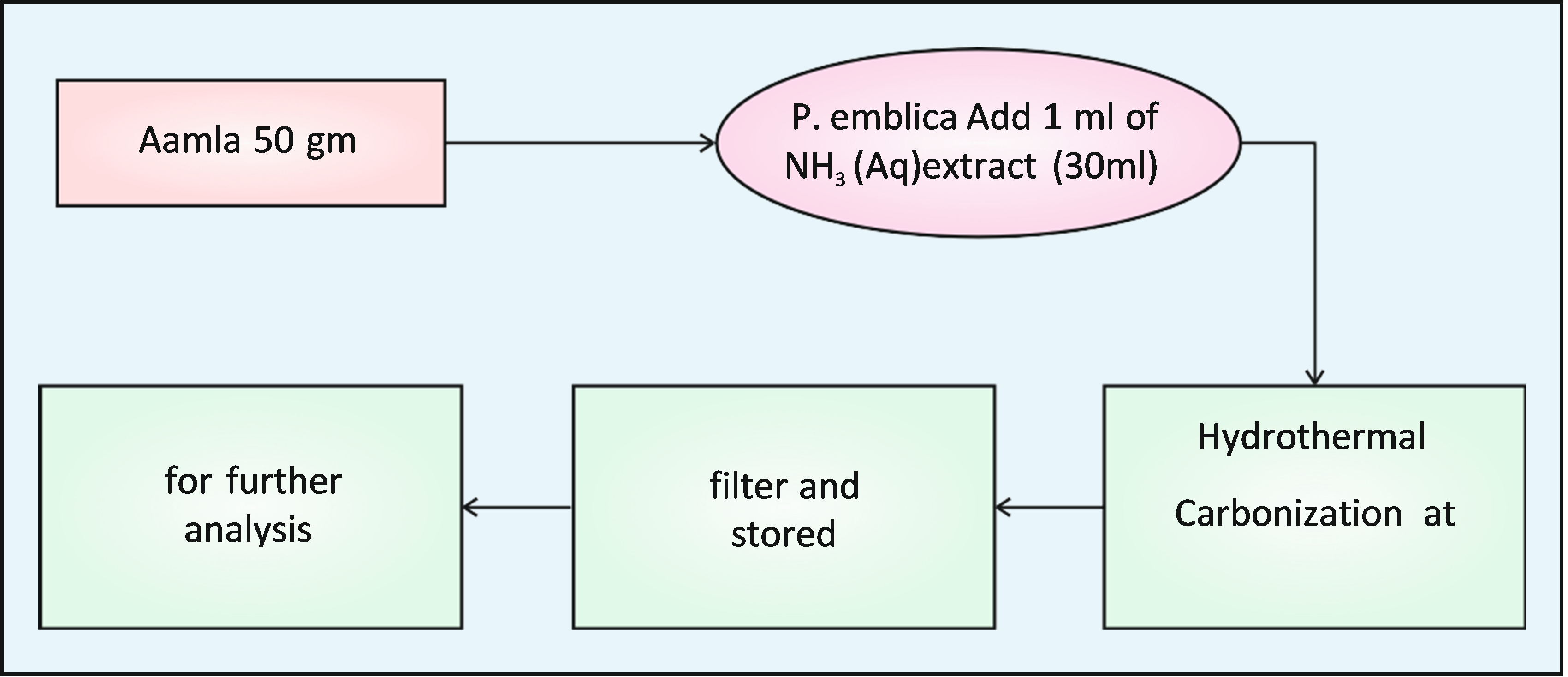
About 50 gm. of P. emblica fruit was thoroughly washed in running water, carefully cut into small, pieces and crushed well mechanically. The prepared amla extract was first filtered by filtration with the help of cotton and then using a Watmann filter paper. The rescutant P. emblica fruit extract was utilized as the biogenic source for the preparation of Carbon quantum dots.


Figure 2: Extract of Phyllanthus Emblica
A simple one-pot hydrothermal Carbonization process was used for the preparation of CQDs from the P. emblica extract. In this process, the mixture of 30ml of the P. emblica extract and 1ml. of aqueous NH3 was taken in a beaker. Add 1ml. H2S04 for acidification of liquid NH3. The soln was kept in a hot air oven for 5 hrs. at 1800C. After the completion of reaction the solution allowed to cool at room temp. (290C). Now the solution was filtered through a whatmann 40 filter paper and centifuge for 15 min at 1000 rpm. The resultant dark brown soln. Containing CQDs was collected and carefully stored in a cool place for further studies
III RESULT & DISCUSSION
Optical Properties:-
When applied green hydrothermal method of Amla fruits has resulted in the colour transformation from pole green to blakish brown indicated the formation of carbon quantum dots. For analyzing the optical characters UV visible spectroscopy is the most valuable tech.
Surface Morphology:-
TEM image of the carbon quantum dots at the range in 20 nm. Carbon quantum dots spread on copper grid is synthesized by green method and spherical in nature .The dimeter of CQDs is 4.08 nm. And size from 1-10 nm.
Structural Analysis:-
The FTIR spectra of Amla fruit and green CQDs synthesized by p. Embilica extract shows broad bands at 3415, 2857, 1706, and 1342 cm-1 that C-H C=O, And C-O-C functional groups respectively.The IR spectra of CQDs had absorption bands at 3536, 2838, 1529, and 146 cm-1 and stretching freqvency of –OH, NH –CH and C-N functional groups respectively.
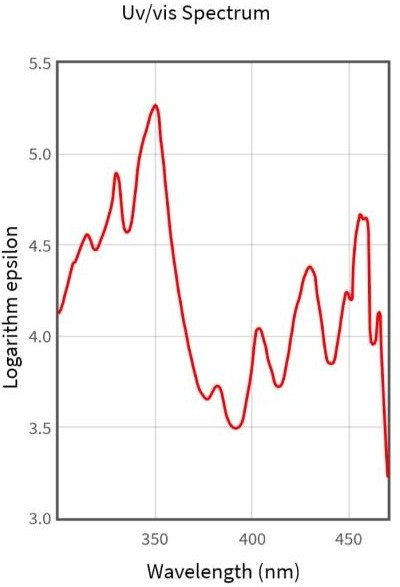
Figure-3: UV – Spectra of P- Emblica Carbon Quantum Dots
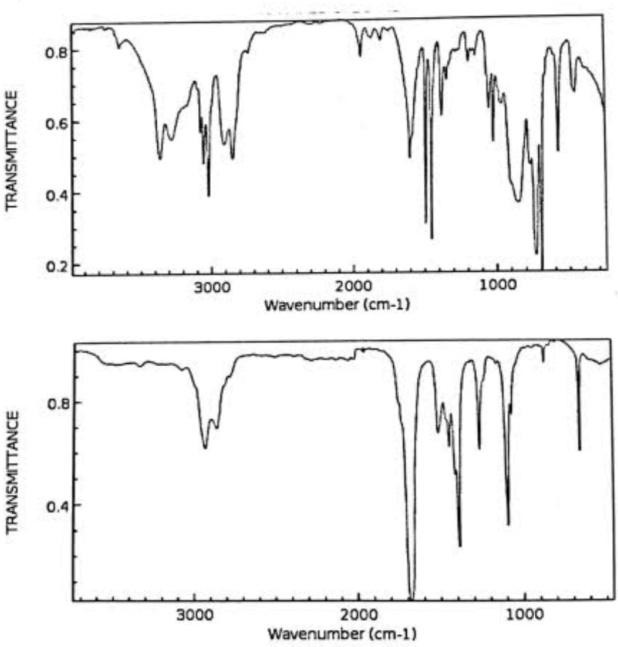
Figure- 4: IR – Spectra of P- Emblica Carbon Quantum Dots
Application of the synthesized CQDs:-
The green synthesized CQDs from gooseberry fruit have dispensability and can show high fluorescence quality and aqueous stability used in bio-imaging applications.So these green synthesized carbon quantum dots used as a fluorescent probe for live cell imaging .For the utilization of the real biological system the bio compatibilitybof a fluorescent probe is a significant factor in biomedical applications.
IV CONCLUSION
In summary, The green carbon quantum dots have been synthesized by surface functionalization using gooseberry fruit amla as a carbon saurce and aqueous ammonia as a solvent.This is hydrothermal one step clean and economic method.The green synthesized carbon quantum dots have nitrogen and oxygen atom. A high fluorescent activity is shown by the aqueous solution of synthesized CQDs which is depends upon the excitation wavelength and its high photostability. In various optical applications CQDs are successfully employed as a tool because of their fluorescent emission properties excellent stability low cytotoxicity and good compatibility. CQDs also used as a staining agent in bio-imaging applications bio-imaging of HTC-116 human colon cancer cell and also for bio-imaging for others. For writing and drawing without any chemical modification CQDs were used as a fluorescent ink. And for CQD is also used as a ink for thumb impression as it glow when placed in under UV light and does not required any other treatment. CQD is also used as in wet treatment of fingerprint impression as a fluorescent ink. Because this is eco-friendly and user friendly tech. compared to available conventional powder dusting method. Because of good permeability of green CQDs they are also used for bio-logical applications to the whole part of the cells including intended drug.
REFRENCES
[1] Duncan TV, Care, Sun and Color Skin Care. “The challenge of making safe engineered nanomaterial‟s.” NatureNanotechnology 6,683–688(2011).
[2] Khot, Lav R., etal. “Applications of nanomaterial’s in agricultural production and crop protection: are view.”Crop protection 35(2012): 64-70.
[3] Arruda, Sandra Cristina Capaldi, et al. “Nanoparticles applied to plant science: a review.” Talanta 131 (2015):693-705.
[4] Hand ford Caroline E etal. “Implications of nanotechnology for the agri-food industry opportunities benefits and risks.” Trends in Food Science & Technology 40.2(2014):226-241.
[5] Ali mohammadi, Mohammad, et al. “Physiological responses induced in tomato plants by a two component nanostructural system composed of carbon nanotubes conjugated with quantum dot sand its in vivo multimodal detection.” Nanotechnology 22.29(2011):295101.
[6] Khodakovskaya, M.V.; Silva, K.D.; Biris, A.S.; Dervishi,E.;Villagarcia, ACS Nano. 2012, 6, 2128-2135.
[7] Zheng L, Hong F, Lu S, Liu C, Biol. Trace Element Res.104:83-91 (2005).
[8] Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR, Environ. Toxicol. Chem. 27:1825- 1851 (2008).
[9] R. Rossetiiand L. Brus, J. Phys. Chem., 1982, 86, 4470–4472.
[10] A. I. Ekimov, A. L. Efros and A. A. Onushchenko, Solid State Commun., 1985, 56, 921–924.11.A.Henglein, Chem. Rev., 1989,89, 1861–1873M. G. Bawendi, M. L. Steigerwald and L.E. Brus, Annu. Rev. Phys. Chem., 1990,41,477–496
[11] B.O. Dabbousi, J. Rodriguez-Viejo, F.V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K.F. Jensenand M.G. Bawendi, J. Phys. Chem. B, 1997, 101,9463– 9475.
[12] C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715.15.W.C. W. Chanand S.Nie, Science, 1998,281,2016–2018.
[12] M. Bruchez Jr, M. Moronne, P.Gin, S. Weissand A.P.Alivisatos, Science, 1998, 281, 2013–2016.
[13] A.P. Alivisatos, Nat. Biotechnol., 2004, 22, 47–52.
[14] U. Resch- Genger, M. Grabolleand S. Cavliere -Jaricot, Nat. Methods, 2008, 5,763–775.
[15] A.H. Poliandri, J.P. Cabilla, M.O. Velardez, C.C. Bodoand B.H. Duvilanski, Toxicol. Appl. Pharmacol., 2003,190,17–24
[16] R.A. Hardman, Environ. Health Perspect., 2006,114,165–172.
[17] S. Satarugand M.R. Moore, Environ. HealthPerspect.,2004,112,1099–1103.
[18] F.R. Baptista, S.A. Belhout, S. Giordani and S.J. Quinn, Chem.Soc.Rev., 2015, 44,4433‐4453.
[19] A. Cayuela, S. Benítez‐Martínez and M.L. Soriano, Tr AC Trend. Anal. Chem., 2016, DOI: http://dx.doi.org/10.1016/j.trac. 2016. 02.016.
[20] Geim, A.K.; Novoselov, K.S., Therise of graphene. Nat Mater 6, 183-191 (2007).
[21] Late DJ, Ghosh A, Subrahmanyam KS, PanchakarlaL S, Krupanidhi SB, Rao CNR. Solid State Communications. 150: 734-738(2010).
[22] X. Y. Xu, R. Ray, Y.L. Gu, H.J. Ploehn, L. Gearheart, K.Rake and W.A. Scrivens, J.Am.Chem.Soc.,2004,126,12736‐12737.
[23] Y.F. Wang and A.G.Hu, J. Mater. Chem. C, 2014, 2, 6921‐6939.H.P.Liu, T. Yeand C.D. Mao, Angew. Chem. Int.Edit., 2007, 46, 6473‐6475.
[24] Albrecht, M.A., Evans, C.W. and Raston, C.L.(2006).Green chemistry and the health implications of nanoparticles. Green Chem. 8, 417-420.
