pp: 16-24
Deepak Kumar Gupta1*, Rajveer Singh Rajaura1, Nakuleshwar Dut Jasuja2, Kananbala Sharma3
1,3Centre for Converging Technologies, University of Rajasthan, Jaipur
2School of Science, Suresh Gyan Vihar University, Jaipur- 302017, Rajasthan, India
3 Semiconductor and Polymer Science Lab, Department of Physics, University of Rajasthan
*Corresponding Author, Email: deepak.nanoconverge@gmail.com
Abstract
Graphene oxide (GO) is a biocompatible nano-material possessing good antibacterial activity. In GO sp2– bonded carbon atoms arranged in a single layered two-dimensional hexagonal pattern and the edges of nanoparticles contain functional exogenous oxygen bearing groups such as hydroxyl, carbonyl, carboxylic and epoxy group, which makes the atomic layer hydrophilic and expand interlayer distance. They have tremendous application in catalysis, composite materials, solar energy, biosensors and biomedical application. The present work deigned to prepare graphene oxide nanoparticles (GONPs). Antimicrobial activity of prepared NPs is investigated against Bacillus subtilis, Staphylococus epidermidis, Pseudomonas aeruginosa and Enterobacter aerogenes. Characterizations of GONPs were examined by Raman Spectra, SEM (scanning electron microscope). SEM studies indicate that the GONPs of size range 50-60 nm. The GONPs had shown maximum zone of inhibition (ZOI) i.e.12 mm in E. aerogenes
Keyword: GONPs, SEM, Raman Spectra, Antibacterial activity.
Introduction
Graphene oxides have currently emerged as a new carbon- based nanoscale particle that provides an alternative path to graphene due to its extraordinary properties like large specific surface area volume ratio and low production cost (Stankovich, et al., 2006; Zhao, et al., 2010). It’s a single layered material made up of oxidize graphite which available in large quantities at inexpensive prices. Structurally, the graphene oxide is very similar to a graphene sheet with its base having oxygen containing groups such as hydroxyl, carbonyl groups, carboxylic and epoxy group (Lerf, et al., 1998, Ivey, et al., 2008). Since these groups have a high affinity to water molecules i.e. hydrophilic and can be easily dissolved in water and other solvents allows it to be informally deposited on to wide ranging substrates in the form of thin films, which makes it potentially useful for micro-electronics (Eda, et al., 2008).
Recently, graphene oxide has gained more attention because it is functionalized easily with fluorescent probe and other compatible biomolecules (Liu, et al., 2010 and 2011; Pham, et al., 2011). These unique properties of GO make it a promising nanomaterial for bioapplication. Industrially produced graphene oxide could be used for wide range of application such as solar cell (Xinjuam, et al., 2012), hydrogen storage(Wang, et al., 2007), transparent conductive films (Park, et al., 2010), Polymer composite (Zhang, et al., 2009, Eda and Chhowalla, 2009), Paper like materials (Dikin, et al., 2007), biomedicine (Mohanty and Berry, 2008; Yousefim, et al., 2012; Bykkam, et al., 2013), fabricating nanoelectronic devices (Bunch, et al., 2007), energy storage devices (Liu, et al., 2010), biosensors (Prabhakar, et al., 2008; Lu, et al., 2009; Zhou, et al., 2010; Yancai, et al., 2013), catalysis (Chauhan, et al., 2011) and transparent electrodes (Zhang, et al., 2010).
In recent years tremendous attention has been drawn regarding the antimicrobial activity of graphene and its functionalized NPs (Akhavan and Ghaderi, 2009; Zhu, et al., 2010; Hu et al., 2010, Shen, et al., 2010; Das, et al., 2011; Guo, et al., 2012; Shaobin, et al., 2012). Bykkam et al. recently synthesized GONPs that shows very good antibacterial activity against Klebseilla and Staphylococus bacterial species (Bykkam, et al., 2013)
In the present study, GONPs were prepared and their antibacterial activity was investigated against Gram-positive and Gram-negative bacteria i.e. Bacillus subtilis, Staphylococus epidermidis, Pseudomonas aeruginosa and Enterobacter aerogenes.
Materials and Methods
Synthesis of Graphene Oxide Nanoparticles
Graphene oxide (GO) was synthesized according to Hummer’s method (Hummers, 1958) by using Graphite flakes, sodium nitrite, Hydrogen peroxide (30%), Sulphuric acid (70%), and Potassium permanganate (99%). KMnO4 (9 g) was added in portions to a cooled (0 °C) solution of conc. H2SO4 (69 ml) containing graphite (3 g) and NaNO3 (1.5 g). The mixture was stirred at room temperature for 5 days. Distilled water (138 ml) was added slowly to the reaction mixture while the temperature was kept well below 98 °C for 3 h. The resultant bright-yellow suspension was diluted and a solution of H2O2 (6 ml) was added drop wise. The reaction mixture was centrifuged and washed to remove the remaining salts. The wet GO was dewatered by vacuum drying (50 °C). Aqueous colloids of single layer graphene oxide nanosheets were produced by exfoliation of graphite oxide dispersed in deionized water with ultrasonication. For the preparation of Reduced Graphene Oxide, Graphite oxide (75 mg) was dispersed in water (75 ml) with sonication and sodium borohydride (600 mg) was added to the GO dispersion after the pH being adjusted to 9–10 with 5 % sodium carbonate solution. The mixture was then kept at 80 °C for 1 h under constant stirring. During reduction, the dispersion turned from dark brown to black accompanied by out gassing. This mixture was then centrifuged at 10,000 rpm for 15 minutes and washed 3-4 times with distilled water to remove all the impurities. Which result in the synthesis of graphene oxide nanoparticles (Chauhan, et al., 2011).
Characterization
The surface morphology and microstructure of the prepared GONPs was characterized by Carl ZEISS-SMART SEM (Scanning Electron Microscopy) and Raman spectroscopy (Dilor XY-800 spectrometer), using 514 nm wavelength of an argon-ion laser.
Antimicrobial Assay of GONPS
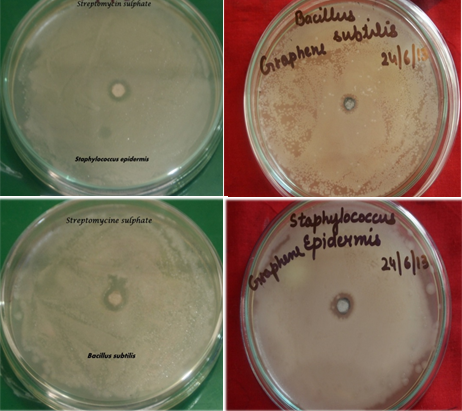
Antimicrobial assay of synthesized nanoparticles was done on Bacillus subtilis, staphylococcus epidermidis, Pseudomonas aeroginosa and Enterobacter aerogenes by disc diffusion method. Sterile 6mm of diameter of whatman no.1 filter paper disks were prepared by applying 100 μg/ml of synthesized GONPs and streptomycin as standard for bacteria. The disc was dried in hot oven until it gets fully dry. On the other hand suspension of bacteria was made and O.D. was taken of the suspension after growth of 24 hrs. For bacteria standard O.D. is between 0.1-0.8 at 600nm. Whereas, O.D. of E. aerogenes, B. subtilis, P. aeroginosa and S. epidermidis found to be 0.8, 0.6, 0.75 and 0.5 respectively. The test organisms were sub-cultured in nutrient broth media for 12 hrs. These cultures were used for antimicrobial assay .The nutrient agar media was poured in sterile petriplates under sterile conditions and left to solidify. About 100μl of bacterial suspension was uniformly spread on media and disks were placed in the centre of the petriplate. One standard antibiotic disc was placed in a petriplate as a control. The culture plate were incubated at 37o C and the zone of inhibition (ZOI) was measured after 24hrs (Shamelie, et al., 2012).
Results and Discussion
Raman Analysis
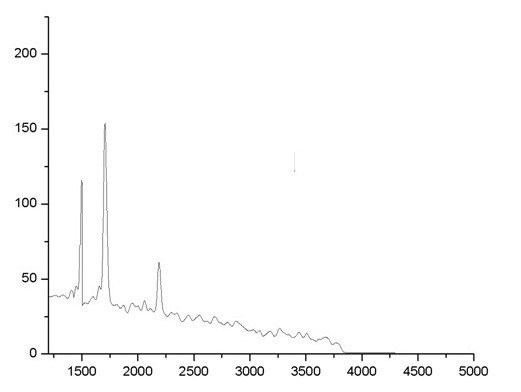
Raman spectroscopy is a non-destructive technique that is widely used to obtain structural information about carbon-based materials. The main features in the Raman spectra of graphitic carbon based materials are the G and D peaks and their overtones. The first-order G and D peaks, both arising from the vibrations of sp2 carbon, appear at around 1580 cm-1 and 1350 cm-1, respectively. The G peak corresponds to the optical E2g phonons at the Brillouin zone center resulting from the bond stretching of sp2 carbon pairs in both, rings and chains. The D peak represents the breathing mode of aromatic rings arising due to the defect in the sample. The D-peak intensity is therefore often used as a measure for the degree of disorder. The shift and shape of the overtone of the D peak, called as 2D peak around 2680 cm-1, can be correlated to the number of graphene layers (N). The 2D peak is attributed to double resonance transitions resulting in the production of two phonons with opposite momentum. Further, unlike the D peak, which is Raman active only in presence of defects, the 2D peak is active even in the absence of any defects. Typical Raman spectrum of GO obtained at an excitation wavelength of 532 nm is shown in Fig 1. The prominent D peak at ~1392 cm-1 with an intensity comparable to the G peak ~1592 cm-1 along with their large band width are indicative of significant structural disorder in GO.
Scanning Electron Microscopy Analysis
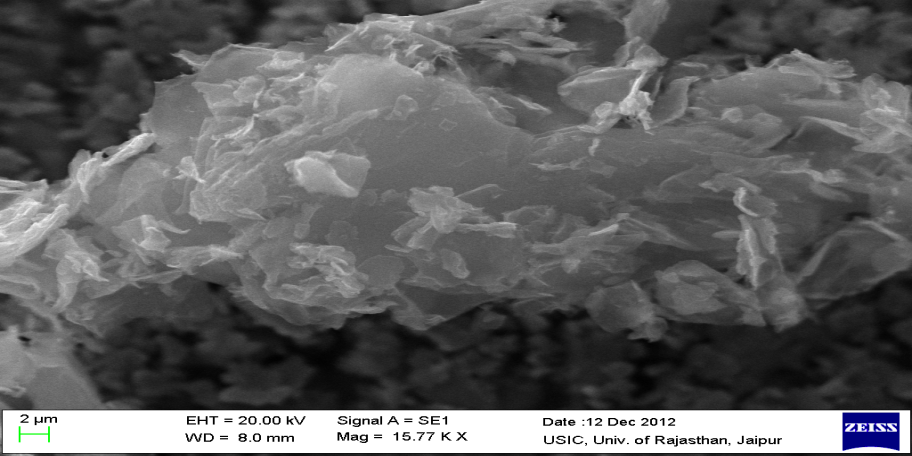
The SEM micrographs of synthesized GO with different scale bars are given in Fig.2. From the figure, it can be observed that graphene oxide has layered structure, which affords ultrathin and homogeneous graphene films. Such films are folded or continuous at times and it is possible to distinguish the edges of individual sheets, including kinked and wrinkled areas.
Antibacterial Activity Studies
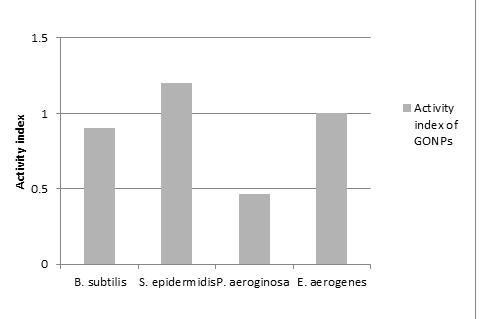
The observation was recorded and summarized in Table 1. As shown in Table 1, GONPs samples generally exhibit antibacterial activity. The activity of sample was identified by the formation of zone of inhibition at 37˚C after 24hr. The presence of zone of inhibition conformed inhibitory antibacterial activity of GONPs. The zone surrounding the sample is clear that shows complete zone of inhibition. The space surrounding the complete zone of inhibition is partial zone of inhibition where the activity decreases than complete zone of inhibition.
The study revealed that GONPs had shown highest toxicity against S. epidermidis (12 mm ZOI) and E. aerogenes (12 mm ZOI) Whereas, lowest on B. subtilis (9 mm ZOI) and P. aeroginosa (7 mm ZOI) as compared to standard drug i.e. streptomycin (100µg/ml). GONPs could bind on the surface of bacterial cell through hydrogen bonds between the bacteria’s lipopolysaccharides and the exogenous oxygenated functional groups of GO. Hence, GONPs could prevent the nutrient uptake process of the bacterial cell (Das et al 2011). GONPs could induce cellular damage of bacterial cell and the cytoplasm flowing out due to either oxidative stress or physical disruption (Hu, et al., 2010).
Conclusion
Graphene oxide also possesses non-toxological effects and hence can be widely used in medicinal research. Due to antimicrobial activity of graphene oxide, they can also be employed in dental resin composites, bone cement, ion exchange fibers and coatings for medical devices, biosensors and nano-biotechnology research. The results showed that graphene oxide nanoparticles presented good antibacterial activity effective against common human pathogenic microorganism.
Raman Spectra:
Nano Particle and their antibacterial activity graph
Figure 1. Raman Analysis of graphene oxide nanoparticles
Nano Particle and their antibacterial activity image
Figure 2. SEM Analysis of graphene oxide nanoparticles
Table 1: Zone of inhibition of antimicrobial activity for graphene oxide as compared to streptomycin
| Bacteria | GONPs(100µg/ml) | Streptomycin(100µg/ml) |
| Bacillus subtilis | 9 mm | 10 mm |
| staphylococcus epidermidis | 12 mm | 10 mm |
| Pseudomonas aeroginosa | 7 mm | 15 mm |
| Enterobacter aerogenes | 12 mm | 12 mm |
Graphene Image
Figure 4. Antibacterial activity of 100µg/ml graphene oxide nanoparticles and antibiotics. Comparative graphical representation of inhibition zones.
Activity Index
Figure 5. Activity index of AgNPs and GONPs compared with Streptomycin
Reference
Ahmad, M. B., Lim, J. Y., Shameli, L., Ibrahim, N. A., Tay, M. Y., and Chieng, B. W. 2012. Antibacterial activity of silver bionanocomposites synthesized by chemical reduction route, Bin Ahmad et al. Chemistry Central Journal. 6:101.
Ahmad, N. and Sharma, S. 2012. Biosynthesis of silver nanoparticles from biowaste pomegranate peels, International Journal of Nanoparticles. 5: 185-195.
Akhavan, O.and Ghaderi, E. 2009. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation, J. Phys. Chem. C. 113 (47): 20214–20220
Anil. K., Suresh, Dale A Pelletier, Wei Wang, Ji-Won Moon, Baohua Gu, Ninell P Mortensen, David P Allison, David C Joy, Tommy J Phelps, Mitchel J Doktycz. 2010. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ Sci Technol. Jul 1; 44 (13):5210-5.
Bunch, J. S., van der Zande A. M., Verbridge S. S., Frank I. W., et al. 2007. Electromechanical Resonators from Graphene. Science 315- 490.
Bykkam, S., Rao, V. K., Chakra, C. H. S. and Thumgunta, T. 2013. Synthesis and Characterization of Graphene Oxide and Its Antimicrobial Activity Agents Klebseilla and Staphylococcus. Int. J. Adv. Biotech. & Res. 1(4): 142-146.
Chaloupka, K., Malam, Y., Seifalian, A. M. 2010. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 28:580–588.
Chauhan, S. M. S. and Mishra, S. 2011. Use of Graphite Oxide and Graphene Oxide as Catalysts in the Synthesis of Dipyrromethane and Calix (4) Pyrrole. Molecules. 16: 7256-7266.
Das, M. R., Sarma, R. K., Saikia, R., Kale, V. S., Shelke, M. V., Sengupta, P. 2011. Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surfs B. 83:16–22.
Das, M. R., Sarma, R. K., Saikia, R., Kale, V.S., Shelke, M. V. and Sengupta, P. 2011. Synthesis of Silver Nanoparticles in an Aqueous Suspension of Graphene Oxide Sheets and Its Antimicrobial Activity. Col. & Sur. B: Bio. 83: 16-22.
Dikin, D. A., Stankovich, S., Zimmey, E. J., Piner, R. D., et al. 2007. Preparation and characterization of graphene oxide paper. Nature. 448: 457-460.
Eda, G. and Chhowalla, M. 2009. Graphene-based Composite Thin Films for Electronics. Nano Lett. 9: 814-818.
Eda, G., Fanchini, G. and Chhowalla, M. 2008. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat Nanotechnol. 3: 270-274.
Evanoff, D. D., Chumanov, G. 2005. Synthesis and optical properties of silver nanoparticles and arrays. Chemphyschem. 6:1221–1231.
Frattini, A., Pellegri, N., Nicastro, D., de Sanctis, O. 2005. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 94: 148.
Guo, J., wang, R., Tjiu, W. W., Pan, J. and Liu, T. 2012. Synthesis of Fe Nanoparticles @ Graphene Composites for Environmental Application. J. Harzar. Mater. 63-73.
Hu, W., Peng, P., Luo, W., Lv, M., Li, X., Li, D., Huang, Q. and Fan, C. 2010. Graphene-based antibacterial paper, ACS Nano 7: 4317-23.
Hummers, O. WSRE. 1958. Preparation of Graphitic Oxide. J Am hem Soc. 80: 1339.
Ivey, K.N., Muth, A., Arnold, J., et al. 2008. Microrna regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2: 219–229.
Lerf, A., He, H. Y., Forste,r M., Klinowski, J. 1998. Structure of graphite oxide revisited. J Phys Chem B. 102: 4477-4482.
Li, J., Liu, C. y. 2010. Ag/graphene heterostructures: synthesis, characterization and optical properties. Eur J Inorg Chem. 10:1244–1248.
Li, Y., Huang, F., Chen, J., Mo, T., Li, S., Wang, F., Feng, S., Li, Y. 2013. A High Performance Enzymes-free Glucose Sensor Based on the Graphene-CuO Nanocomposites. Int. J. Electro. Sci. 8: 6332-6342.
Liu, C., Yu, Z., David, N., Aruna, Z. and Jang Bor, Z. 2010. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 10: 4863–4868.
Liu, S. et al., 2012. Lateral Dimension Dependant Antibacterial Activity of Graphene Oxide Sheets. Langmuir. 28: 12364-12372.
Liu, Y., Liu, C., Liu, Y.2011. Investigation on fluorescence quenching of dyes by graphite oxide and grapheme. Applied Surface Science. 257: 5513-5518.
Liu, Y., Yu, D., Zeng, C., Miao, Z., Dai, L. 2010. Biocompatible graphene oxide-based glucose biosensors. Langmuir. 26: 6158– 6160.
Lu, G., Ocola Leonidas, E. and Chen, J. 2009. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology. 20:445502.
Mohanty, N. and Berry, V. 2008. Graphene-based singal-becterium resolution biodevice and DNA transistor: Interfacing graphene derivative withnanoscale and microscale biocomponents. Nano Lett. 8: 4469-4476.
Nath, S. S., Chakdar, D. and Gope, G. 2007. Synthesis of CdS and ZnS quantum dots and their applications in electronics, Nanotrends- A journal of nanotechnology and its application. 02.
Pal, S., Tak, Y. K., Song, J. M. 2007. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli_Appl. Environ. Microbiol. 73: 1712.
Panacek, A., Kvitek, L., Prucek, R., Kolar, M., Vecerova, R., Pizurova, N., Sharma, V.K., Nevrcna, T. J., Zboril, R. 2006. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B., 110:16248–16253.
Park, S., Mohanty, N., Sui, D. et al. 2010. Flexible, magnetic and electrically conductive graphene/Fe3O4 Paper and its application for magnetic controlled switches. J Phys Chem C. 1141: 17465-174771.
Pham, T. A., Choi, B. C., Lim, K.T., Jeong, Y. T. 2011. A simple approach for immobilization of gold nanoparticles on graphene oxide sheets by covalent bonding. Applied Surface Science. 257:3350-3357.
Prabhakar, N., Arora, K., Singh, H. and Malhotra Bansi, D. 2008. Polyaniline Based Nucleic Acid Sensor. J. Phys. Chem. B. 112:4808-4816.
Rai, M., Yadav, A., Gade, A. 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 27:76–83.
Shameli, K., Ahmad, M. B., Jazayeri, S. D., Shabanzadeh, P., Sanpour, P., Jahangirian, H. and Gharayebi, Y. 2012. Investigation of antibacterial activities of Silver nanoparticlesprepared via green method. Chemistry Central Journal. 6:73.
Sharma, V. K., Yngard, R. A., Lin, Y. 2009. Silver nanoparticles: green synthesis and their antimicrobial activities, Colloid Interface Sci. 145: 83.
Shen, J., Shi, M., Li, N., Yan, B., Ma, H., Hu, Y., Ye, M. 2010. Facile synthesis and application of Ag-chemicallyconverted graphene nanocomposite. Nano Res. 3: 339-349.
Sileikaite, A., Puiso, J., Prosycevas, I., Tamulevicius, S. 2009. Investigation of Silver Nanoparticles Formation Kinetics During Reduction of Silver Nitrate with Sodium Citrate. Materials Science. 15(1).
Soukupova, J., Kvitek, L., Panacek, A., Nevecna, T., Zboril, R. 2008. Comprehensive study on surfactant role on silver nanoparticles (NPs) prepared via modified Tollens process. Mater Chem Phys. 111:77–81.
Stankovich, et al. 2006. Graphene-based composite materials. Nature. 422: 282-286.
Wang, X., Zhi, L. and Mullen, k. 2007. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 8: 323-327.
Xinjuam, Liu. et al., 2012. UV-assisted Photocatalytic Synthesis of ZnO-reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity in Reduction of Cr (VI). Chem. Eng. J. 183: 238-243.
Yousefia, M., Hassanzadehb, S. M., Tavakoliniaa, F., Tavakoliniaa, F., Sheshmania, S., Khaliliana, F., Tehrania, R. M., Rahimnejada, S., Arab, M. 2012. Synthesis and Characterization of Silver Nanoparticles/ Graphene Oxide Nanocomposites an Antimicrobial Agent. 4th Int. Confer. Nano. 1358-1360.
Zhang, L., Zhao, S., Tian, X. and Zhao, X. 2010. Layered Graphene Oxide Nanostructures with Sandwiched Conducting Polymers as Supercapacitor Electrodes. Langmuir. 26(22): 17624–17628.
Zhang, Y., Lin, L., Feng, Z., Zhou, J., Lin, Z. 2009. Fabrication of a PANI/Au nanocomposite modified nanoelectrode for sensitivedopamine nanosensor design. Electrochimica Acta. 55: 265–270.
Zhao, X., Zhang, Q., Hao, Y., Li, Y., Fang, Y., Chen, D. 2010. Alternate multilayer films of poly (vinyl alcohol) and exfoliated graphene oxide fabricated via a facile layer-by-layer assembly. Marcomolecules. 43:9411-9416.
Zhou, K., Zhu, Y., Yang, X., Luo, J., Li, C., Luan, S. A. 2010. novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochimica Acta. 55: 3055–3060
Zhu, B. Y., Murali, S., Cai, W., Li, X., Suk J. W.,, Potts, J. R., Ruoff, R. S. 2010. Graphene and Graphene Oxide: Synthesis, Properties and Applicatin. Adv. Mater. XX: 1-19.
