Volume 6, Issue 1, 2020, pp. 19-23
Tarun Patodia1,2, S. Katyayan1, Balram Tripathi2, a)
1 Department of Physics, Suresh Gyan Vihar University, Jaipur.
2Department of Physics, S.S. Jain Subodh P.G.(Auto.) College, Rambagh Circle, Jaipur
a)balramtripathi1181@gmail.com
ABSTRACT
In this study, GO has been synthesized by Hummer’s technique. XRD, FESEM, Raman and FT-IR spectrophotometer have been used for characterization of GO. XRD spectra confirms crystalline structure of GO. Raman spectra confirms the electronic structure of GO. FESEM image showing layered surface of GO. Due to its dramatic properties GO would be useful as cathode material for Li-S battery.
Keywords: Graphene oxide, Layered surface, Cathode
1.INTRODUCTION
Graphene has a remarkable molecule thick structure, magnificent conducting, mechanical, and warm characteristics [1]. Subsequently, investigation for its use in energy storage devices & gadgets, catalysis, sensors, and vitality transformation and capacity and so on. For these reasons, the large scale manufacturing of such materials at minimum expenses is fundamental prerequisites [2-6].
Moreover, GO is processable and can be manufactured or self-collected into naturally visible materials having controlled structures for pragmatic applications [7]. GO is the forerunner of rGO; therefore, it assists in controlling the different characteristics of rGO [8-13]. Schafhaeul discussed GO in 1840 [14] followed the same by Brodie in 1859 [15]. In this technique, graphite was mixedwith KClO3 and responded in raging HNO3 at 60ºCelsius for 92 hrs. Staudenmaier modified Brodie technique by supplanting around two thirds of raging HNO3 with concentrated H2SO4 and including KClO3 in different parts. This little alteration empowers the general response in a solitary vessel; along these lines rearranging the blend strategy for 4 days [16]. The most significant technique for the combination of GO was created by Hummers what’s more, Offeman in 1958 (Hummers strategy) [17]. In this method, graphite oxidation was accomplished by treatment of graphite powders in a concentrated H2SO4 arrangement containing KMnO4, 0.5 equivalent load of NaNO3. The Hummers strategy, at least, has three significant points of interest over past strategies as the response can be finished inside a couple hours. KClO3 was supplanted by KMnO4 to improve the response security, staying away from the development of unstable ClO2 and the utilization of NaNO3 as opposed to smoldering HNO3 wipes out the arrangement of corrosive mist [18].
For a protected situation, it is required to give more consideration for the advancement of lithium sulfur battery having high energy density and good cycle life. Presently, carbon materials have been progressively tried for upgradation in cathode materials for lithium sulfur batteries [19-20]. Sheets of graphene oxide comprise carbon molecules having sp3 arrangement mostly and is a coated compound improved by C, H, O in factor proportion to make GO hydrophilic and henceforth frames watery colloids by straightforward arrangement procedure to encourage a gathering of naturally visible structure [21-22]. It is likewise nature biocompatible utilized in vitality capacity, bio sensor and malady discovery and so forth. Conduction capacity of GO relies upon oxidation and amalgamation in compound. Likewise, epoxide bunches containing oxygen permit GO to scattered in water and other natural solvents. Sheets of GO are precisely solid films having a huge number of little pieces. Functionalization of GO can be done from multiple points of view contingent upon the wanted application like additives for electrode material of Lithium sulfur batteries, fuel cell, sensors or as medication conveyance material [23-25].
Here we report modified Hummer’s method using charcoal activated in place of graphite flakes for synthesis of GO having different stirring time and temperature. Further X-ray diffraction analysis, FT-IR, Raman spectroscopy, and FESEM were used for characterization of synthesised graphene oxide.
- EXPERIMENTAL SECTION
2.1. Materials
Charcoal activated (Rankem), Sulphuric acid (98.08%, Fisher scientific), Sodium nitrate (Rankem), Ice cubes, Potassium permanganate (>99%, CDH), distilled water, Hydrochloric acid (35.0%, Rankem) and hydrogen peroxide (30% Rankem) used for synthesis of Graphene oxide.
2.2. GO Preparation
Hummer’s method [26-27] was used in which 3 gm Charcoal activated powder was mixed into 50mL concentrated sulphuric acid and 2gm of sodium nitrate in a 1000mL flask placed in an ice-bath (0-8ºC) with 2 hrs stirring with help of magnetic stirrer, then 6 gm of potassium permanganate (KMnO4) as a oxidising agent was added moderately to suspension obtained in such a manner that temp should not be increased more than 10ºC and stir the suspension for 1 hrs and ice bath is then removed and kept stirring for 2hrs with temp below 35ºC until it becomes pasty brownish.100mL distilled water was then loaded into suspension and heated at 95ºC for 30 minutes as a result brown colour occurs again deionized water is mixed in the suspension and stirring is further allowed for half hour. Finally, 20 mL H2O2 was added and stirred for 1 hr appearance of yellow colour indicates the formation of graphene oxide and washed with distilled water and hydrochloric acid to remove metal impurities several times till pH value reaches 7 and dried under vacuum for 24 hrs.
- RESULTS AND DISCUSSION
3.1. X-Ray Diffraction (XRD)
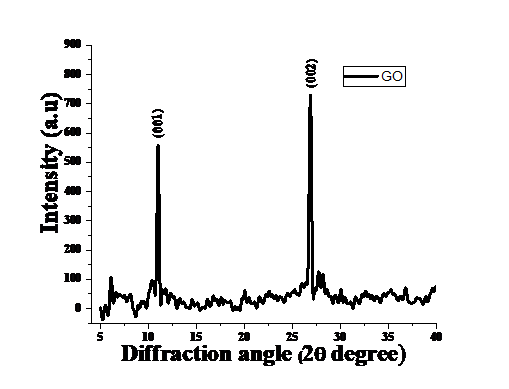
Figure 1. XRD spectrum of GO
XRD plot of synthesized GO has been recorded using panalytical’s powder X-Ray diffractometer having CuKα as source (λ=1.5406 Ȃ) within 2Ɵ=5°-40° at the scan speed 1°/min. Powder X-ray software has been used for analysing the structure and Debye Scherer [28] formula(eq.1) used to calculate the crystallite size which on further calculation comes out 30 nm and d-spacing obtained 8.6135nm with help of Braggs law.
D=kλ/βcosθ……………………………………………….(1)
Where k is scherrer constant (0.9), λ is X-ray wavelength (.15406nm), θ is Bragg diffraction angle, β is FWHM of the XRD peak at diffraction angle θ.
Occurrence of hydroxyl, epoxy and carbonyl groups in GO [29]. GO synthesised showed a very strong peak at 2 = 10.9º, which is in agreement with previous studies [30-31]. XRD results initially confirms synthesis of GO.
3.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
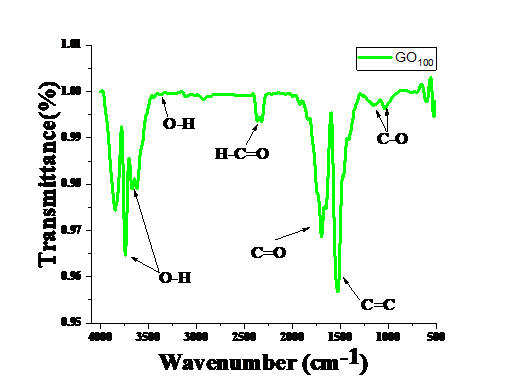
Figure 2. FT-IR spectrum of GO
FT-IR measurements have been performed by using Bruker alpha spectrometer with Zinc Selenide crystal to investigate the functional and structural groups. Results obtained showed adsorption bands for the aromatic C=C (1531 cm−1), carboxyl C=O (1689 cm−1), alkoxy C–O (1027 cm−1), epoxy C–O (1173 cm−1), and hydroxy –OH (3391 cm−1) groups. Groups containing oxygen, such as C=O and C–O, reveals that graphite has been oxidized into GO [32-37].
3.3 Raman Spectroscopy Analysis
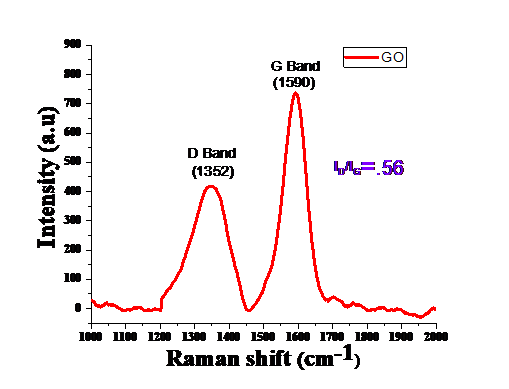
Figure 3. Raman spectrum of GO
Raman measurements have been performed in the backscattering geometry using 514.5 nm line (power5200 mW) from an argon ion laser was used for observation of structural modification via oxidation process.Fig.3 reveal two characteristic bands at 1352 cm-1 (D band) and1590 cm-1 (G band) for GO [38]. From plot we can observe that broadening of both bands implies that average size of sp2 domain is more [35]. Relative intensity ratio ID/IG obtained after oxidation is 0.56. Further GO isn’t an absolutely sp2 framework yet an exceptionally scattered one with a critical sp3 content. Along these lines, in spite of the standard sp2 materials, the abatement of deformities in GO would create an expansion of the D/G proportion. This is on the grounds that there would be more sp2 C particles encompassing the deformities [39].
3.4 Field Emission Scanning Electron Microscope (FESEM)Analysis
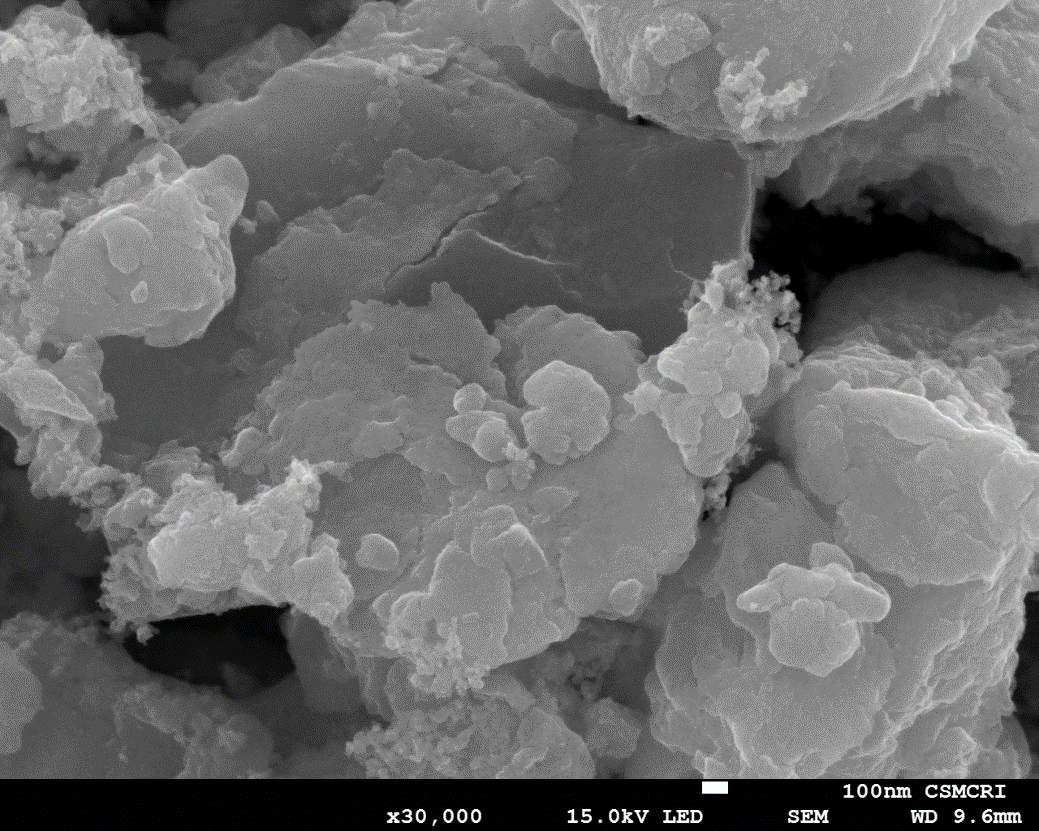
Figure 4. FE-SEM image of GO
Surface morphology of the synthesized GO has been analysed by FE-SEM (JSM-7100F) as shown in image (Fig. 4) confirms some agglomerated layered structure of graphene oxide.
- CONCLUSION
The particle size of synthesized GO obtained from XRD spectrum is of the order of 30 nm. The bonding behaviour obtained from FT-IR showing the aromatic C=C (1531 cm−1), carboxyl C=O (1689 cm−1), alkoxy C–O (1027 cm−1), epoxy C–O (1173 cm−1), and hydroxy –OH (3391 cm−1) groups. Raman spectra shows ratio of ID/IG is of the order of 0.56. FESEM image shows ridged surface of graphene oxide. Based on these evidences authors suggests that graphene oxide would be useful as an additive material for electrode of Li-S battery.
ACKNOWLEDGEMENT
This work is financially supported by the grant from SERB-DST New Delhi under ECR scheme (Grant No. ECR/000655/2017). The author would also thank to Dr. A. S. Verma (Department of Physics, BanasthaliVidyapith, India) for XRD characterization, Dr. V. Kulshrestha (Department of Physics, CSIR-Bhavnagar, India) for FESEM analysis, Dr. S. K. Jain (School of Basics Science, Manipal University Jaipur, India) for FTIR spectroscopy of the composites prepared.
REFERENCES
[1] Novoselov KS, Fal’ko VI, Colombo L, Gellert PR, Schwab MG, Kim K, (2012) A roadmap for graphene.
Nature;490(7419):192–200.
[2] Weiss NO, Zhou H, Liao L, Liu Y, Jiang S, Huang Y, et al., (2012) Graphene: an emerging electronic
material. Adv. Mater;24(43):5782–825.
[3] Huang C, Li C, Shi G., (2012) Graphene based catalysts. Energy Environ Sci; 5(10):8848–68.
[4] Liu Y, Dong X, Chen P., (2012) Biological and chemical sensors based on graphene materials. Chem. Soc.
Rev ;41(6):2283–307.
[5] Sun Y, Wu Q, Shi G., (2011) Graphene based new energy materials. Energy Environ Sci;4(4):1113–32.
[6] Wassei JK, Kaner RB., (2013) Oh, the places you’ll go with graphene. Acc. Chem. Res.10.1021/ar300184v.
[7] Li C, Shi G., (2012) Three-dimensional graphene architectures. Nanoscale ;4(18):5549–63.
[8] Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, et al., (2010) Graphene and graphene oxide: synthesis,
properties, and applications. Adv. Mater;22(35):3906–24.
[9] Wu Z-S, RenW, Gao L, Liu B, Jiang C, Cheng H-M., (2009) Synthesis of high-quality graphene with a pre-
determined number of layers. Carbon ;47(2):493–9.
[10] Zhang L, Li X, Huang Y, Ma Y, Wan X, Chen Y., (2010) Controlled synthesis of few-layered graphene
sheets on a large scale using chemical exfoliation. Carbon;48(8):2367–71.
[11] Zhao J, Pei S, Ren W, Gao L, Cheng H-M., (2010) Efficient Preparation of large-area graphene oxide sheets
for transparent conductive films. ACS Nano;4(9):5245–52.
[12] Xu Y, Sheng K, Li C, Shi G., (2011) Highly conductive chemically converted graphene prepared from
mildly oxidized graphene oxide. J Mater Chem;21(20):7376–80.
[13] Li Y, Umer R, Samad YA, Zheng L, Liao K., (2013) The effect of the ultra-sonication pre-treatment of
graphene oxide (GO) on the mechanical properties of GO/polyvinyl alcohol composites. Carbon; 55:321–7.
[14] C. Schafhaeutl, (2009), On the combinations of carbon with silicon and iron, and other metals, forming the
different species of cast iron, steel, and malleable iron3, 570-590.
[15] Brodie BC., (1859) On the atomic weight of graphite. Philos Trans R Soc London, 14:249–59.
[16] Staudenmaier L., (1898) Methods of representation the graphite acid.1898;31(2):1481–7.
[17] Hummers WS, Offeman RE., (1958) Preparation of graphitic oxide. J. Am. Chem. Soc;80(6):1339.
[18] Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, et al., (2010) Improved synthesis of
graphene oxide. ACS Nano;4(8):4806–14.
[19] Hari Mohan, E.; Sarada, B. V.; Venkata Ram Naidu, R.; Salian, G.; Haridas, A. K.; Appa Rao, B. V.; Rao,
- N., (2016) Graphene-Modified Electrodeposited Dendritic Porous Tin Structures as Binder Free Anode
for HighPerformance Lithium-Sulfur Batteries. Electrochim. Acta, 219, 701−710.
[20] Shanshan Li, Bo Jin, Xiaojie Zhai, Huan Li, and Qing Jiang[a], (2018) Review of Carbon Materials for
Lithium‐Sulfur Batteries chemistry select,3,2245-2260
[21] H. C. Schniepp, J. L. Li, M. J. McAllister, et.al I. A. Aksay, (2006) Functionalized single graphene sheets
derived from splitting graphite oxide, J.Phys. Chem. B 2006, 110, 8535.
[22] S. Pei, H. M. Cheng, (2012) The reduction of graphene oxide Carbon N. Y. 2012, 50, 3210.
[23] K. S. Novoselov, V. I. Fal, L. Colombo, P. R. Gellert, M. G. Schwab,K. Kim, V. I. F. ko, L. Colombo,
- R. Gellert, M. G. Schwab, K. Kim, (2013) A roadmap for graphene. Nature, 490, 192.
[24] Y. Si, E. T. Samulski, (2008), Synthesis of Water Soluble Graphene, Nano Lett., 8, 1679.
[25] M. Hirata, T. Gotou, M. Ohba, (2005) Thin-film particles of graphite oxide 1: High-yield synthesis and
flexibility of the particles, Carbon N. Y. 2005, 43, 503.
[26] S. Abdolhosseinzadeh, H. Asgharzadeh, H. Seop Kim, (2015) Fast and fully-scalable synthesis of reduced
graphene oxide Sci. Rep,5, 10160.
[27] Z. Fan, K. Wang, T. Wei, J. Yan, L. Song, B. Shao, (2010) An environmentally friendly and efficient route
for the reduction of graphene oxide by aluminum powder, Carbon N. Y.,48, 1686.
[28] Uwe Holzwarth and Neil Gibson, (2011), The Scherrer equation versus the ‘Debye–Scherrer equation’
Nature nanotechnology, 6,1
[29] Fan Z.J., Kai W., Yan J., Wei T., Zhi L.J., Feng J., Ren Y.M., Song L.P., Wei F., (2011) Facile Synthesis of Graphene
Nanosheets via Fe Reduction of Exfoliated Graphite Oxide. ACS Nano; 5:191–198.
[30] D. C. Marcano, D. V. Kosynkin, J. M. Berlin et al., (2010) Improved Synthesis of Graphene Oxide ACS
Nano, vol. 4, no.8, pp. 4806– 4814, 2010.
[31] Z. J. Fan, K. Wang, T. Wei, J. Yan, L. P. Song, and B. Shao, (2010) An environmentally friendly and efficient route
for the reduction of graphene oxide by aluminum powder,” Carbon, Vol 48, no 5, pp 1670- 1692.
[32] V. Loryuenyong, K. Totepvimarn, A. Buasri,et.al (2013), Preparation and Characterization of Reduced
Graphene Oxide Sheets via Water-Based Exfoliation and Reduction Methods,10.1155,923403
[33] S. Thakur, N. Karak, (2012) Green reduction of graphene oxide by aqueous phytoextractsCarbon N. Y.
2012, 50, 5331.
[34] D. R. Dreyer, S. Park, C. W. Bielawski, and R. S. Ruoff, (2010) The chemistry of graphene oxide,”
Chemical Society reviews, vol. 39, no. 1, pp. 228–240.
[35] M. El Achaby, F. Z. Arrakhiz, A. Qaiss, et.al., (2012) Piezoelectric β-polymorph formation and properties
enhancement in graphene oxide – PVDF nanocomposite films Appl. Surf. Sci, 258, 7668.
[36] M. D. Stoller, S. Park, Z. Yanwu, J. An, and R. S. Ruoff, (2008) Graphene-based ultracapacitors,” Nano
letters, vol. 8, no. 10, pp. 3498–3502.
[37] F. Thema, M. Moloto, E. Dikio, (2012) Synthesis and Characterization of Graphene Thin Films by Chemical
Reduction of Exfoliated and Intercalated Graphite Oxide, Journal of chem., id 150536,6, vol. 2013
[38] S. Gurunathan, J. Woong Han, A. A. Dayem, V. Eppakayala, J.h.Kim,(2012) Oxidative stress-mediated
antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa
International Journal of Nanomedicine 2012:7 5901–5914
[39] Jianguo Song,Xinzhi Wang,and Chang-Tang Chang,(2014) Preparation and Characterization of Graphene
OxideHindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 276143,1-6
