Vol. 7 Issue 1 Page No. 11-25
Review article
Paramjeet K., Bhasin Sheetal, Dubey Arti, Nagar Hariom* and Sharma Dipak
School of Applied Sciences, Suresh Gyan Vihar University, Jaipur, India
Abstract
Coumarin was first isolated as a natural product in 1820 and named as derived from the French word for the seeds of Tonka bean, coumarou, Dipteryx odorata (Coumarouna odorata) (Leguminosae/ Fabaceae). The structure of heterocyclic compound is found in most of the drugs that is the reason why heterocycles are of vital interest in the pharmaceutical and agrochemical science. In the metabolism of most of the living cells various heterocyclic compounds plays important role. In addition, a wide variety of pharmacological, biological and physiological activities are also exhibited by O-based heterocyclic aromatic coumarin compounds. There are following pharmacological activities for example; anti-cancerous, anti-coagulant, anti-oxidant, anti-HIV, anti-inflammatory and anti-bacterial which are exhibited by coumarin. Various sources of natural coumarins and their biological activities are compiled in the present review article.
Keywords
Natural coumarin Source
biological activities
*Corresponding author’s e-mail addresses: hariom.nagar@mygyanvihar.com
Received: 25 July 2020; Accepted: 27 Nov 2020; Published: 25 Jan 2021
Introduction
Heterocyclic compounds have an important part in the pharmaceutical industry for the search of effective bioactive agents. More than 90% of pharmaceutical drugs have a heterocyclic ring, e.g. penicillin, quinoline, which contains heterocyclic structures are used as analgesics, antibiotics and anti-tumor drugs. Many of the heterocyclic compounds were isolated from natural resources and various synthetic methods were also developed for their synthesis. Major sources of complex heterocyclic compounds are the microorganisms which are used as antibiotics in various infectious diseases. The coumarins are classify under the benzopyrone family of heterocyclic compounds in which 6-membered α-pyrone ring fused with benzene ring and generally occurs in various natural products as a benzo derivative.
Coumarin and its derivative compounds represent one of the most active class that exhibit various types of biological activity such as antibacterial (Al-Amiery et al. 2014; 2016; 2016), antifungal (Al-Amiery et al. 2012), anti-inflammatory (Al-Amiery et al. 2013), anticoagulant (Al-Amiery et al. 2011), anti-HIV (Al-Ayed 2011) and antitumor agents (Al-Azawi et al. 2016). Coumarins are extensively employed as additives in cosmetics, food and perfumes (Al-Majedy et al. 2016) and as pharmaceuticals and optical brighteners (Al-Majedy et al. 2014) and would disperse in fluorescent and laser dyes (Al-Majedy et al. 2016).
Sources of Coumarins
Coumarins are found in significant quantities in plants, and its presence in microorganisms and animal sources has also been detected. Coumarin is naturally present in many plants, especially in high amounts in the tonka bean (Dipteryx odorata), vanilla grass (Anthoxanthum odoratum), sweet woodruff (Galium odoratum), mullein (Verbascum spp.), sweet grass (Hierochloe odorata), cassia cinnamon (Cinnamomum cassia), sweet-clover (Melilotus sp.), deer tongue (Dichanthelium clandestinum), and the leaves of many cherry blossom tree (Prunus genus). Cinnamomum zeylanicum the true cinnamon (Ceylon cinnamon) contains little amount of coumarin (Leal 2000; Lino 1997).
The important dietary sources of coumarins are species of Apiaceae and Rutaceae family. Besides fruits and vegetables some essential oils and beverages are also the sources of coumarins. These essential oils are used as flavoring agents in foods. Table 1 is showing certain literature reports on dietary sources of coumarins. Aroma of coumarin was sweet, aromatic, creamy vanilla bean odour with nutlike natures. In human diet the main source of coumarin is cinnamon which is considered as a spice and comes from the dried bark of C. cassia/C. aromaticum and Cinnamomum verum. In preparation of desserts, cakes, candy, etc. and in many curries and other dishes Cinnamon is widely used as flavoring agents.
Table 1: Dietary sources of coumarins
| S. No. | Family | Food source | Reference |
| 1 | Apiaceae | Aniseed, carrots, celery, coriander, cumin, fennel and parsley | (Jaw-Ming et al. 2008) |
| 2 | Rutaceae | Citrus and some other fruits such as Bael | (Trease and Evans 2009) |
| 3 | Lauraceae | Chinese cinnamon oil, cinnamon bark oil | (Choi et al. 2001) |
| 4 | Lamiaceae | lavender oil | (Bourgaud et al. 2006) |
| 5 | Oleaceae | olive oil | (Rosselli et al. 2009) |
| 6 | Ericaceae
(Heath family) |
Bilberry | (Nykanen 1984) |
| 7 | Rosaceae
(Rose family) |
Cloudberry | (Nykanen 1984) |
| 8 | Theaceae | Black and green tea | (Sonnenberg et al.1995) |
| 9 | Rubiaceae. | coffee | (Sonnenberg et al.1995) |
| 10 | Others | Wine | (Sonnenberg et al.1995) |
TDI (Tolerable daily intake) of coumarin derivatives are 0.1 mg/kg (Lake 1999) and the maximum permissible level for food and beverages is 2 mg/kg (European Council 1988). Estimated value of coumarins diet is about 0.02 mg/kg/day and estimation of TAMDI (Theoretical added maximum daily intake) of coumarin through food is 4.085 mg/day (0.07 mg/kg/day) (AFC 2004).
Many medicinal compounds has been discovered and researchers are trying to improve the therapeutic activity and reduced the toxicity by modify the chemical structure of active part of the drugs. Coumarin is a natural product isolated from plant and it showed various biological activities.
Due to odour strength, tenacity, stability to alkali and relatively cheap price synthetic coumarins are used as a sweetener and fixative in perfume, fragrance enhancers for natural essential oils, blenders in soaps and detergents, aroma enhancers in tobacco and for imparting pleasant odours to industrial products.
According to chemical structure natural coumarins are classified in to six groups-
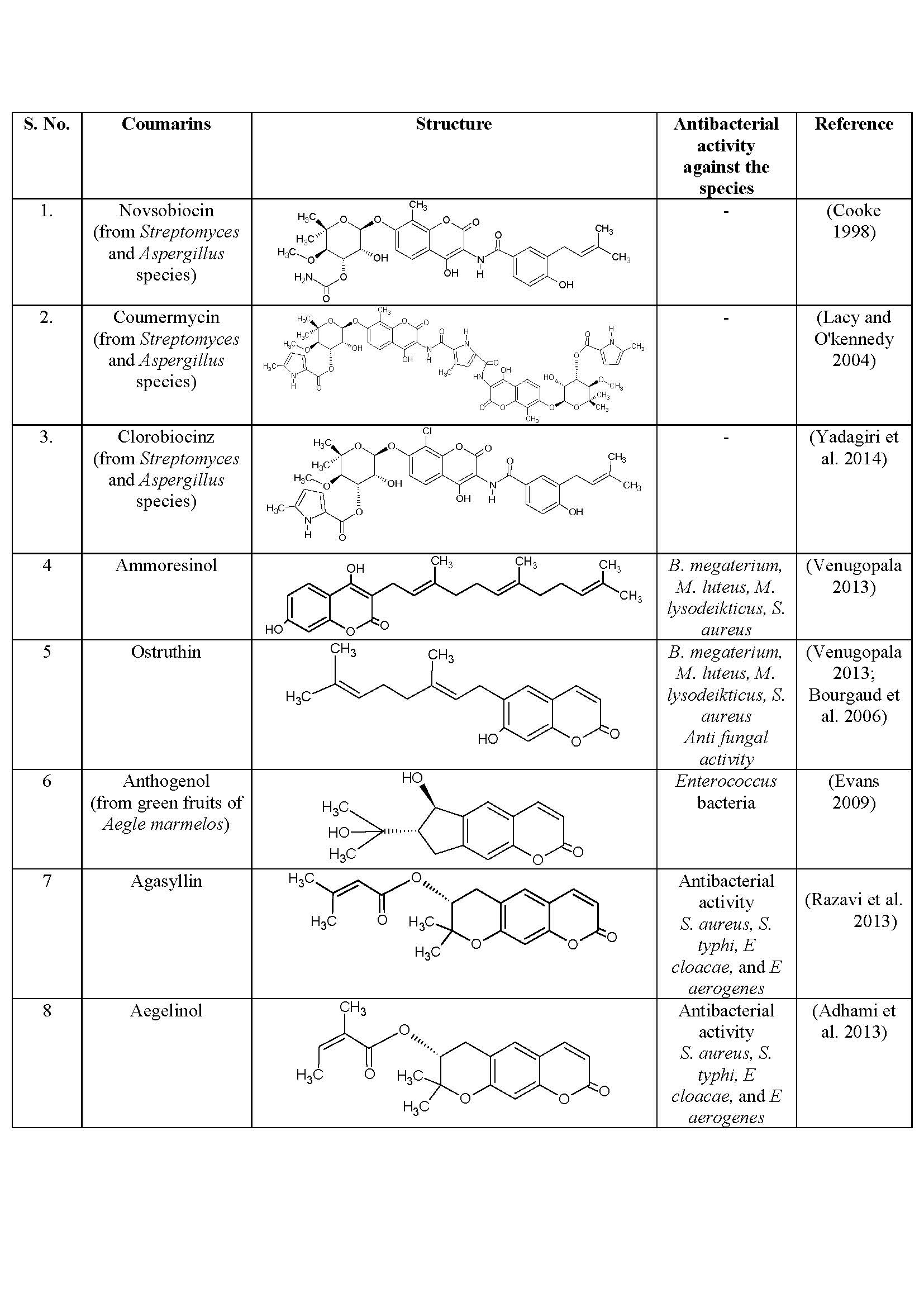
- Simple coumarins that are compounds which formed coumarin derivatives by the reactions; hydroxylation, alkoxylation and alkylation on the benzene ring of coumarin. Ammoresinol, Chartreusin, Coumermysin, Novobiocin and Ostruthin are related to simple coumarins with antibacterial activity and Osthole exhibits anticancer activity (Venugopala 2013).
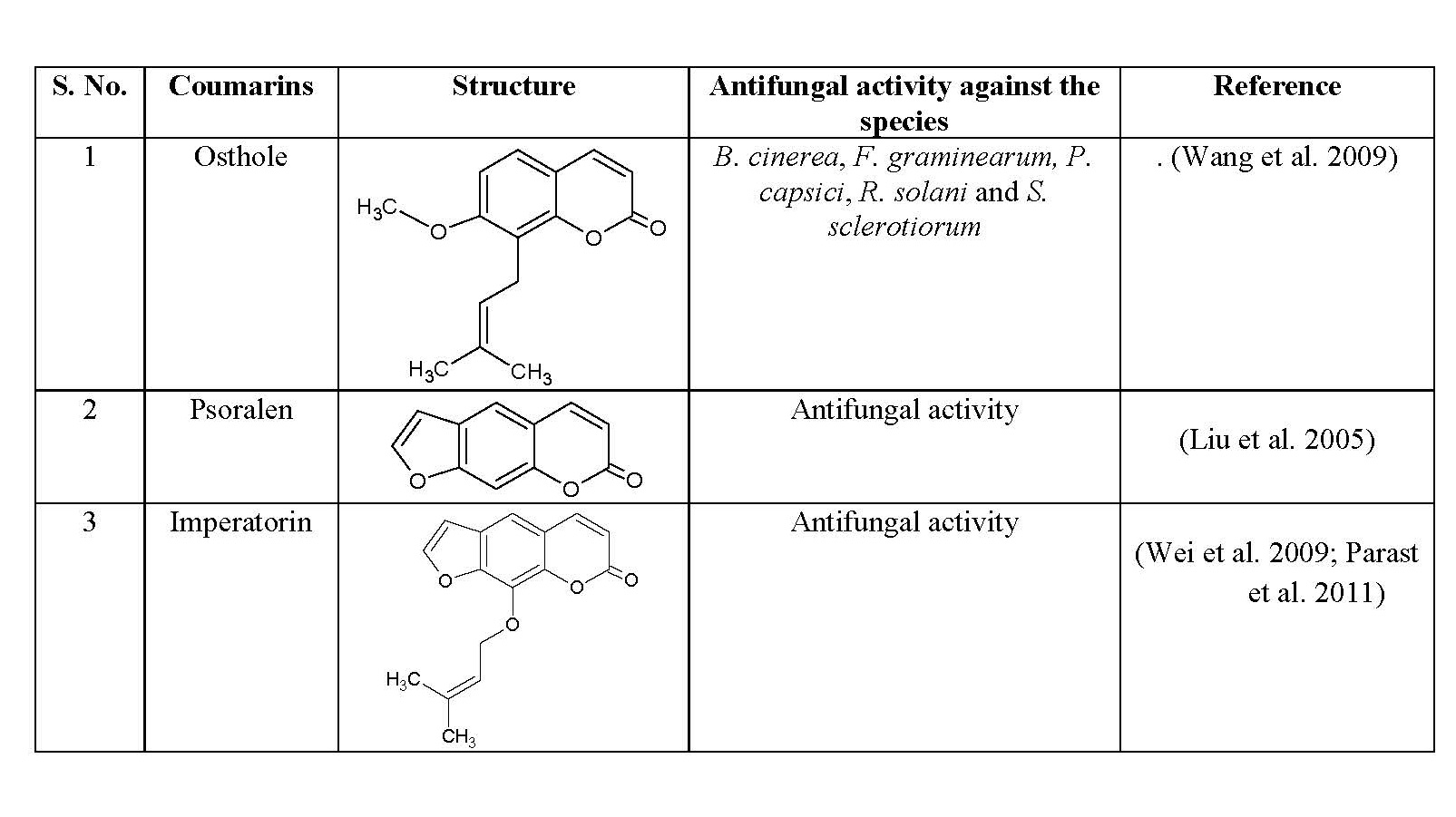
- Furanocoumarins class consists of Bergapten, Imperatorin, Marmalde, Psoralen and other compounds that exhibit antifungal, anti-inflammatory and anti-TB activities.
- Dihydrofurano coumarins consist of five-member furan ring which is attached to the benzene ring of coumarin.
- Pyranocoumarins that have 6-membered pyran ring attached to a benzene ring of coumarin and it is further divided in to two types linear and angular Pyranocoumarins. Linear type consist of Aegelinol benzoate, Agasyllin and Grandivittin (Basile et al. 2009) exhibited anti-bacterial activity and antiviral activity shown by angular type coumarins, Calanolide (A, B, F), Inophyllum (A, B, C, E, P, G) (Mishra et al. 2013) and Pseudocordatolide (Venugopala 2013).
- Phenylcoumarins and 6. Bicoumarins in which substitution in pyrone ring which is generally at position C3 or C4 and have the pharmacological anticoagulant activity.
Isolation of Coumarins
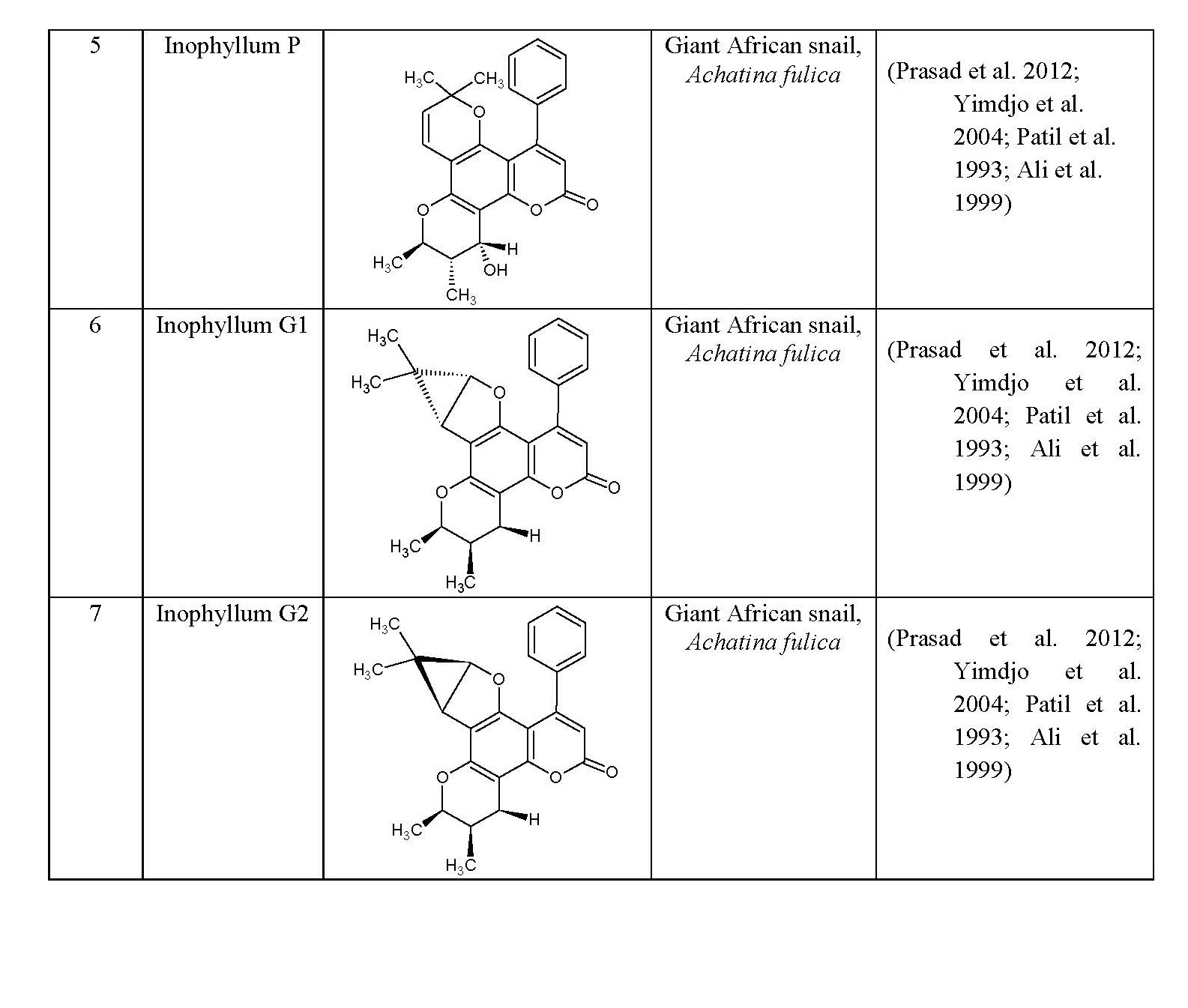
Major coumarin constituents that have been isolated (from plants) are: simple furocoumarins and isofurocoumarins, hydroxycoumarins, biscoumarins, pyranocoumarins, and dihydroisocoumarins (Ribeiro and Kaplan 2002). Several plant species distributed over 40 different families are found which contains coumarins.
Coumarins have been obtained from many plants species distributed over the 40 different families. More than 1300 coumarins have been isolated, which were well distributed in families of Angiospermae, Dicotyledoneae and Monocotyledoneae. In all angiosperm, simple coumarins are the most common, most notably in Asteraceae and Oleaceae, and the furocoumarins and pyranocoumarins were second most prevalent coumarins (Ribeiro and Kaplan 2002).
Skalicka-Wozniak et al. (2018) isolated rare coumarins from fruits of Peucedanum luxurians. They reported that in most cases, with dichloromethane as solvent the fruits gives higher concentrations of most of the investigated coumarins as compared to the aerial parts, with peucedanin compound is identified that it present in great quantity of 4563.94 ± 3.35 mg/100 g concentration. Various gram-negative and gram-positive bacterial strains were used for testing the efficiency of isolated coumarin compounds.
Mustafa et al. (2018) used two methods which are serial soxhlet extraction and kinetic maceration using water, methanol, chloroform, and n-hexane as extraction solvents for the isolation of coumarins from Creston apple seeds. In vitro anti-cancerous (cytotoxic) activity of the mentioned coumarin derivatives was studied by using MTT assay on three cancer cell lines, AMN3, HeLa and MCF-7. The results indicated that against AMN3 and HeLa cell lines, all compounds have higher IC50 values as compared to 5-fluorouracil and against MCF-7 cell line, some compounds have IC50 values which was lower than 5- fluorouracil.
Phytochemicals and nutritional values of plants have been studied for decades and then added as important part of our daily diet. Gogoi (2017) isolated coumarins from Mesua assamica plant which is endemic to Assam in India and reported its cytotoxic (anti-cancerous) activity. Mesua assamica plant belongs to family Clusiaceace, is a slow growing tall evergreen tree. Isolation of various coumarin-derivatives from fruit peels, root and barks, and charactessrization have been done, which are further used as effective anti-cancer agents. Some natural coumarin novobiocin, coumermycin, and clorobiocin were isolated from microbial sources such as Streptomyces and Aspergillus species (Cooke 1998; Lacy and O’kennedy 2004; Yadagiri et al. 2014).
.
Biological Activity of Coumarins
Coumarin and its derivatives are biologically and pharmacologically active compounds with a broad range of properties as antimicrobial, antitumor, anti-HIV, anti-inflammatory, anticoagulant and antioxidant agents. In this review article various biological activities of natural coumarins are highlighted.
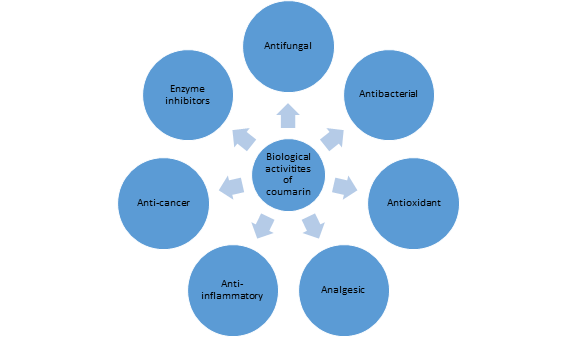
- Antibacterial activity
For prevention or treatment of bacterial infections antibacterial or antibiotics were used and they act either by inhibiting the growth of bacteria or killing the bacteria (Table 2).
Table 2: Antibacterial activity of some natural coumarins
- Antifungal activity
The fungal infection is generally found in hair, nails and skin. Ringworm and athlete’s foot are some common fungal infections. Medications that are used to treat fungal infections are known as antifungal drugs. Certain coumarins, their structures and antifungal activities are compiled in table 3.
Table 3: Antifungal activity of some natural coumarins
- Anti-coagulant and Cardiovascular
Coumarin derivatives possessed cardiovascular and anticoagulant properties. Warfarin (Fig. 1) is a synthetic coumarin analogue that is used as an anticoagulant and is known commercially with the trade name Coumadin.
Coumarins, as a kind of vitamin K antagonists (Schalekamp and de Boer 2010) the most remarkable are phenprocoumon, acenocumarol, and warfarin which are currently using in many countries (Daly 2013; Milatova and Milata 2013). Warfarin is used more oftenly than acenocoumarol due to its longer half-life (36 h), provides theoretically more stable anticoagulation and avoids fluctuations of factor VII that potentially lead to acenocoumarol treatment (half-life 10 h) (Beinema et al. 1998). The structure and anticoagulant activity of warfarin (a coumarins derivative) is also described in the literature (Schalekamp and de Boer 2010; Narayanaswamy et al. 2014).
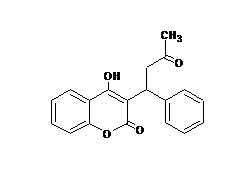
Fig. 1 Structure of Warfarin
- Anti-Viral:
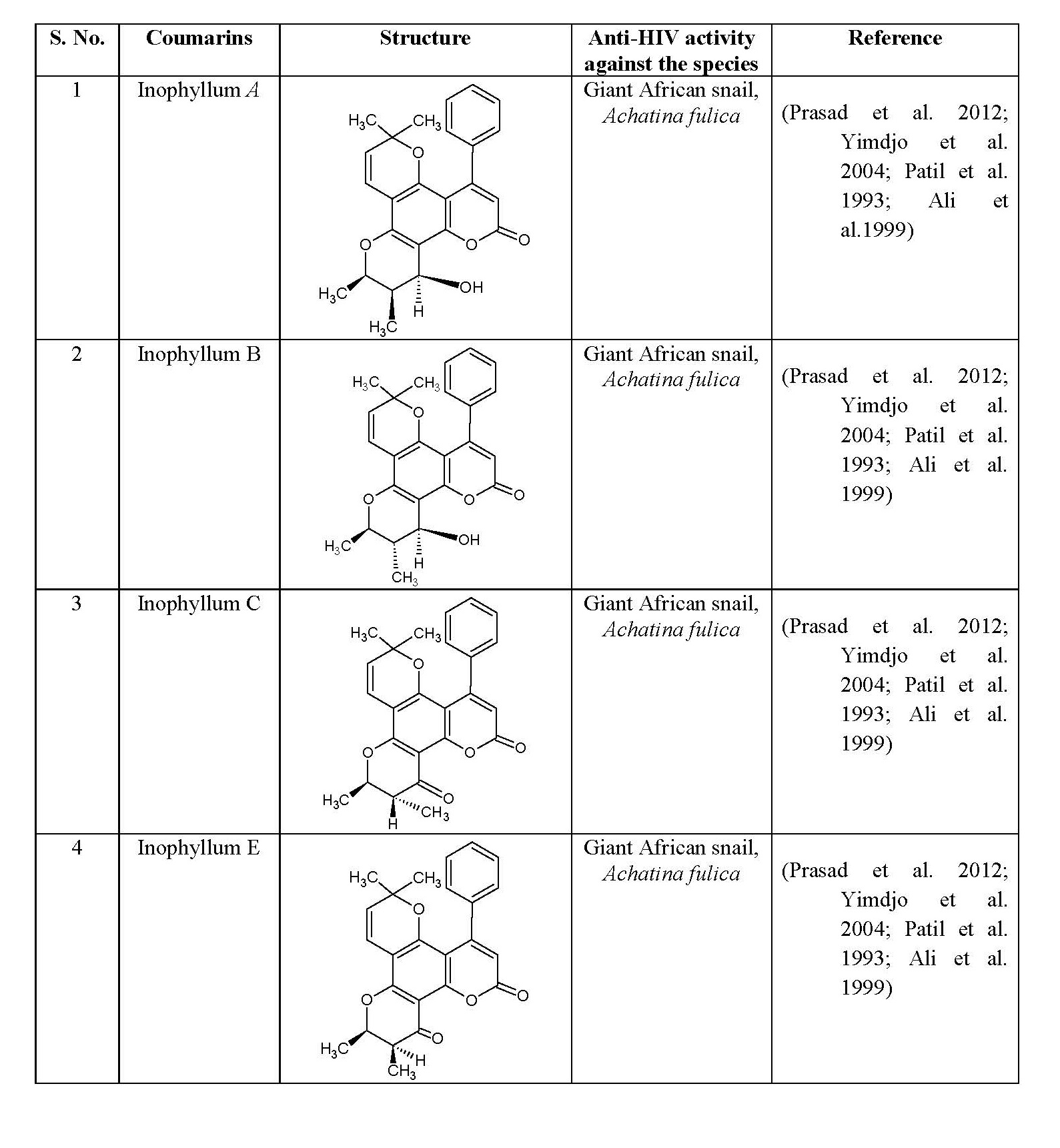
Coumarins showed the antiviral activity against HIV-1 protease (HIV-PR) and HIV-1 integrase. Literature reveals the anti HIV activity of coumarin derivatives, as shown in table 4 herein certain example of anti HIV agents, their structures and activity on certain species are also mentioned in this table.
Table 4: Anti-HIV activity of some natural coumarins
- Antiadipogenic activity
Several natural compounds exhibit strong capacity for decreasing triglyceride accumulation, enhancing lipolysis and inducing apoptosis. The present review paper reported the sources of coumarins and their structure in table 5.
Table 5: Antiadipogenic activity of some natural coumarins
- Anticancer
It is a worldwide serious human health disease and in United States cancer is the second leading disease which causes death. Cancer involves abnormal and autonomous cell growth with potential danger. Cancer cells are carried to the other parts of the body by circulation, where they develop further. This disease caused by various agents such as radiant energy, chemical substances and due to genetic factors. The anticancer drugs which are used in the treatment of this disease act either by modify the growth or killing the cancer cells. Anticancer activity of geiparvarin (a natural coumarin derivative) (Fig. 2) is detailed in literature (Cravotto et al. 2001).
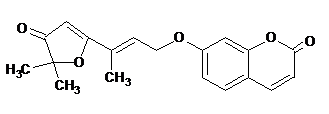
Fig. 2: Structure of Geiparvarin
Conclusion
Above mentioned, review state that coumarin compounds have been widely observed in medicinal chemistry. Coumarins are found as a major class in the plant kingdom. New coumarin derivatives are being discovered or synthesized by various methods in the laboratory. Coumarins exhibit a wide range of biological, pharmacological and physiological profile which leads a strong motivation for more research in this area. As a very large class of compounds coumarins are found in the plant kingdom.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Reference
Adhami H.R., J. Lutz, H. Kählig, M. Zehl, and L. Krenn. 2013. Compounds from gum Ammoniacum with acetylcholinesterase inhibitory activity. Sci. Pharm. 81: 793-806.
AFC. 2004. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to Coumarin. EFSA Journal. 104: 1-36.
Ali M.S., S. Mahmud, S. Perveen, V.U. Ahmad, and G.H. Rizwani. 1999. Epimers from the leaves of Calophyllum inophyllum. Phytochemistry. 50: 1385-1389.
Al-Majedy Y., A. Al-Amiery, and A. Kadhum. 2016. Efficient catalyst one-pot synthesis of 3 7-(aryl)-10,10-dimethyl-10,11-dihydrochromeno[4,3-b] 4 chromene-6,8(7H,9H)-dione derivatives 5 complemented by antibacterial activity. BioMed Res. Int. 5891703: 1-7.
Al-Majedy Y., K. Kadhum, and A.A.H. Al-Amiery. 2014. A synthesis and characterization of some new 4-Hydroxy-coumarin derivatives. Molecules. 19: 11791-11799.
Al-Majedy Y.K., A.A. Al-Amiery, A.A.H. Kadhum, and A.B. Mohamad. 2016. Antioxidant Activities of 4-Methylumbelliferone Derivatives. PLoS ONE. 11: 1-13.
Al-Amiery A.A., Y.K. Al-Majedy, D. Al-Duhaidahawi, Kadhum A.A.H., and Mohamad A.B. 2016. Green Antioxidants: Synthesis and Scavenging Activity of Coumarin-Thiadiazoles as Potential Antioxidants Complemented by Molecular Modeling Studies. Free Radicals and Antioxidants 6: 173-177.
Al-Amiery A.A., Y.K. Al-Majedy, A.A. Kadhum, and A.B. Mohamad. 2014. New coumarin derivative as an eco-friendly inhibitor of corrosion of mild steel in acid medium. Molecules. 20: 366-383.
Al-Amiery A.A., Y.K. Al-Majedy, A.A. Kadhum, and A.B. Mohamad. 2016. Synthesis of new coumarins complemented by quantum chemical studies. Res. Chem. Intermed. 42: 3905-3918.
Al-Amiery A.A., A.A.H. Kadhum, and A.A. Mohamad. 2012. Antifungal Activities of New Coumarins. Molecules. 17: 5713-5723.
Al-Amiery A.A., A.A.H. Kadhum, A.A. Mohamad, A.Y. Musa, and C.J. Li. 2013. Electrochemical study on newly synthesized chlorocurcumin as an inhibitor for mild steel corrosion in hydrochloric acid. Materials. 6: 5466-5477.
Al-Amiery A.A., A.Y. Musa, A. Kadhum, and A. Mohamad 2011. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules.16: 6833-6843.
Al-Ayed A.S. 2011. Synthesis, Spectroscopy and Electrochemistry of New 3-(5-Aryl-4,5-Dihydro-1H-Pyrazol-3-yl)-4-Hydroxy-2H- Chromene-2-One 4, 5 as a Novel Class of Potential Anti-bacterial and Antioxidant Derivatives. Int. J. Org. Chem. 1: 87-96.
Al-Azawi F., S. Al-Baghdadi, A. Mohamed, A. Al-Amiery, T. Abed, S. Mohammed, A.A.H. Kadhum, and A.B. Mohamad. 2016. Synthesis, inhibition effects and quantum chemical studies of a novel coumarin derivative on the corrosion of mild steel in. Chem. Cent. J. 10: 1-9.
Basile A., S. Sorbo, V. Spadaro, M. Bruno, A. Maggio, and N. Faraone. 2009. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules. 14: 939-952.
Beinema M., J.R. Brouwers, T. Schalekamp, and B. Wilffert. 1998. Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon. J. Thromb. Haemost. 80: 899-902.
Bourgaud F., A. Hehn, and R. Larbat. 2006. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 5: 293-308.
Bourgaud F., Hehn A., Larbat R., Doerper S., Gontier E., Kellner S. and Matern U. 2006. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 5: 293-308.
Choi J., K.T. Lee, H. Ka, T. Jung, H.J. Jung, and H.J. Park. 2001. Constituents of the essential oil of the Cinnamomum cassia stem bark and the biological properties. Arch. Pharm. Res. 24: 418-423.
Cooke D. 1998. Studies on the mode of action of coumarins (coumarin, 6-hydroxycoumarin, 7-hydroxycoumarin & esculetin) at a cellular level. PhD thesis, Dublin City University. Dublin, Ireland.
Cravotto G., G.M. Nano, G. Palmisano, and S. Tagliapietra. 2001. An asymmetric approach to coumarin anticoagulants via hetero-Diels–Alder cycloaddition. Tetrahedron: Asymetry. 12: 707-709.
Daly A.K. 2013. Optimal dosing of warfarin and other coumarin anticoagulants: the role of genetic polymorphisms. Arch. Toxicol. 87: 407-420.
European Council. Council Directive of 22 June 1988 on the approximation of the laws of the member states relating to flavourings for use in foodstuffs and to source materials for their production. In: Communities OJotE, editor.1988. 61-66.
Evans W.C. Trease and Evans. 2009. Pharmacognosy. 16th edition ed: Elsevier Ltd.
Gogoi B. 2017. Anti-Cancerous potentiality of coumarins of Mesua assamica (King & Prain) Kosterm. – An Endemic Plant of Assam. IJPPR. 9: 939-942.
Hu H.B., X.D. Zheng, Y.F. Jian, J.X. Liu, and J.H. Zhu. 2011. Constituents of the root of Anemone tomentosa. Arch. Pharm. Res. 34: 1097-1105
Jaw-Ming C.Q., Chiang A.W, and C.B. Lien-Chai. 2008. Immunomodulatory activities of common vegetables and spices of Umbelliferae and its related coumarins and flavonoids. Food Chem. 106: 944-950.
Lacy A., and R. O’kennedy. 2004. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 10: 3797-811.
Lake B.G. 1999. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem. Toxicol. 37: 423-453.
Leal L.K.A.M., A.A.G. Ferreira, G.A. Bezerra, F.J.A. Matos, G.S.B. Viana. 2000. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study. J. Ethnopharmacol. 70: 151–159.
Lino C.S., M.L. Taveira, G.S.B. Viana, F.J.A. Matos. 1997. Analgesic and antiinflammatory activities of Justicia pectoralis Jacq. and its main constituents: coumarin and umbelliferone. PHYTOTHER RES. 11: 211–215.
Liu R., Q. Sun, Y. Shi., and L. Kong. 2005. Isolation and purification of coumarin compounds from the root of Peucedanum decursivum (Miq.) Maxim by high-speed counter-current chromatography. J. Chromatogr. A. 1076: 127-132
Michalska K., and W. Kisiel. 2014. Chemical constituents from Lactuca inermis, a wild African species. Biochem. Syst. Ecol. 55: 104-106.
Milatova E., and V. Milata. 2013. Warfarin – its synthesis and properties in a twenty year retrospective. Ceska Slov. Farm. 62: 111-119.
Mishra K., N. Sharma, D. Diwaker, L. Ganju, and S.B. Singh. 2013. Plant derived antivirals: a potential source of drug development. J. Virol. Antiviral Res. 2: 1-9.
Mustafa Y.F., M.A. Najem, Z.S. Tawffiq. 2018. Coumarins from Creston Apple Seeds: Isolation, Chemical Modification, and Cytotoxicity Study. J. Appl. Pharm. Sci. 8: 049-056.
Narayanaswamy V.K., R.M. Gleiser, K. Kasumbwe, B.E. Aldhubiab, M.V. Attimarad and B. Odhav. 2014. Evaluation of Halogenated Coumarins for Antimosquito Properties. The Sci. World J. 2014: 1-6.
Nykanen I., editor. 1984. The volatile compounds of Hierochloe odorata. Proceedings of the alko symposium on flavour research of alcoholic beverages, Helsinki, Finland: Foundation for Biotechnical and Industrial Fermentation Research. 131-139.
Oshima N., Y. Narukawa, T. Takeda, and F. Kiuchi. 2013. Collagenase inhibitors from Viola yedoensis. J. Nat. Med. 67: 240-245.
Parast B.M., S.V. Chetri, K. Sharma, and V. Agrawal. 2011. In vitro isolation, elicitation of psoralen in callus cultures of Psoralea corylifolia and cloning of psoralen synthase gene. Plant Physiol. Biochem., 49: 1138-1146.
Patil A.D., A.J. Freyer, D.S. Eggleston. 1993. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 36: 4131–4138.
Prasad J., A. Shrivastava, A.K. Khanna, G. Bhatia, S.K. Awasthi, and T. Narender. 2012. Antidyslipidemic and antioxidant activity of the constituents isolated from the leaves of Calophyllum inophyllum. Phytomedecine 19: 1245-1249.
Razavi S.M., G. Imanzadeh, F.S. Jahed, and G. Zarrini. 2013. Pyranocoumarins from Zosima absinthifolia (Vent) Link Roots. RUSS J BIOORG CHEM. 39: 215-217.
Ribeiro C.V., and M.A. Kaplan. 2002. Tendências evolutivas de famílias produtoras de cumarinas em angiospermae. Quim Nova. 25: 533-538.
Rosselli S., A.M. Maggio, and N. Faraone. 2009. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat. Prod. Commun. 4: 1701-1706.
Schalekamp T., and A. de Boer. 2010. Pharmacogenetics of oral anticoagulant therapy. Curr. Pharm. Des. 16: 187-203.
Sonnenberg H., M. Kaloga, N. Eisenbach, and K.K. Fromming. 1995. Isolation and characterization of an angular-type dihydropyranocoumarin glycoside from the fruits of Ammi visnaga (L) Lam (Apiaceae). Z. Naturforsch. C. 50: 729-731.
Venugopala K.N., V. Rashmi, and B. Odhav 2013. Review on natural coumarin lead compounds for their pharmacological activity. Biomed Res. Int. 7: 563-568.
Wang J., L. Liu, Q. Zhang, Z. Zhang, H. Qi, and P. Li. 2009. Synthesized over sulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chem. 114: 1285-1290.
Wei Y., Q. Xie, D. Fisher, and L.A. Sutherland. 2009. Preparative Isolation of Imperatorin, Oxypeucedanin and Isoimperatorin from a Traditional Chinese Herb Using a HSCCC Strategy Based on Optimization of Rapid Flow Rate. Chromatographia 70: 1185-1189
Widelski J., S.V. Luca, A. Skiba, I. Chinou, L. Marcourt, J.L. Wolfender, and K. Skalicka-Wozniak. 2018. Isolation and antimicrobial activity of coumarin derivatives from fruits of Peucedanum Luxurians Tamamsch. Molecules. 23: 1-12.
Yadagiri B., U.D. Holagunda, R. Bantu, L. Nagarapu, C.G. Kumar, S. Pombala, and B. Sridhar. 2014. Synthesis of novel building blocks of benzosuberone bearing coumarin moieties and their evaluation as potential anticancer agents. Eur. J. Med. Chem. 79: 260-265.
Yimdjo M.C., A.G. Azebaze, A.E. Nkengfack, A.M. Meyer, B. Bodo, and Z.T. Fomum, 2004. Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry. 65: 2789-2795.
Zhu L.X., and X.J. Hou. 2008. Investigation on the Quality of Musalais Grape Wine [J]. Liquor-Making Science & Technology, 6: 014.
