pp. 45-59
Dr. Sunita Ojha
Assistant Professor,
School of Applied Science,
Suresh Gyan Vihar University,
Jaipur-302017
Contact No. 9664284669, 8133880357
Email id: sunita.ojha@mygyanvihar.com; ojhasunita2017@gmail.com
ABSTRACT
Silk is known as the Queen of textiles due to its elegance, class and comfort. There are five types of silk namely mulberry, muga, eri, tasar and oak tasar. The caterpillar producing these silks are called silkworms. Silkworms depend on their host plant for food source. These plants are also medicinally important. Traditionally, crude extract of these plants are used as herbal medicines. These extract contain an array of compounds having biological activities such as antimicrobial, antioxidant, anticancer and anti-inflammatory activities acting synergistically. Generally, these compounds are plant secondary metabolites and considered as a storehouse for potent drugs due to their diverse structure and biological activities. Most of these compounds are phenolic compounds, alkaloids, flavonoids, saponins, etc. In this article, we have reviewed the primary host plants of mulberry and non-mulberry silkworm for their in vitro and in vivo biological activities.
Keywords: Host plant, secondary metabolites, silkworm, therapeutic, biological activity
- INTRODUCTION
Seri-resource is a dynamic resource which includes silkworm, its host plants, their symbiotic and parasitic organisms. Silkworms feed on plant leaves during larval period of its life cycle. It has a preference for some plants as host which are classified into primary, secondary and tertiary host plants. These plants also have medicinal properties which are generally attributed to the secondary metabolites produced by them. Generally, plants produce these compounds in response to stress conditions either abiotic or biotic. Various abiotic stresses that act on plants are shown in Figure 1. On the other hand, biotic stress includes pathogen attack, herbivory, etc.
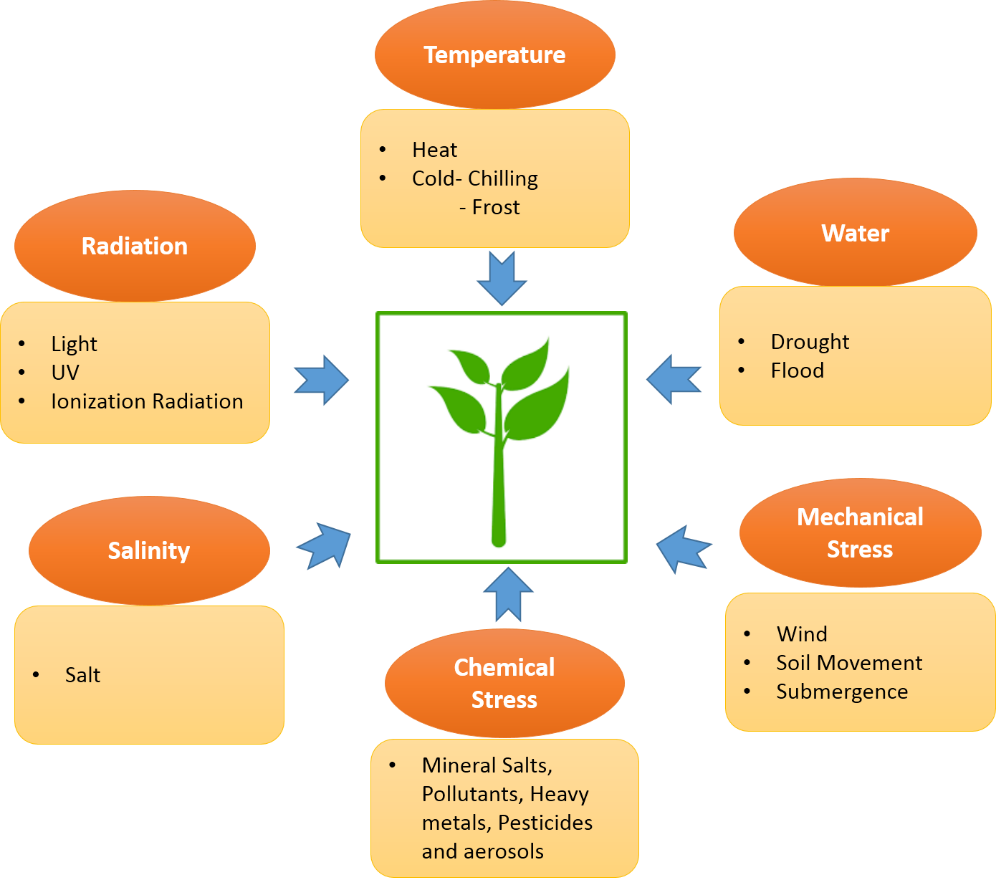
Figure 1. List of abiotic stress acting on plants
The secondary metabolites have no role in the primary functions of plants like growth, development or reproduction. But they are important for their defense against herbivores and pathogens. Moreover, nectar also contains secondary metabolites which filter out the nectar robbers from natural pollinators [1]. Plant produces some volatile compounds by the process of secondary metabolism which is responsible for the host specificity of silkworms.
The metabolites are structurally classified into terpenoids, alkaloids and phenolics. They are further subdivided into glycosides, saponins, and tannins. Terpenoids constitute larger component of phytochemicals including carotenoids, steroids and gibberellic acids [2]. Structurally they are composed of isoprene units linked in a head-to-tail fashion. They are classified based on the numbers of units linked. They are synthesized from acetate via mevalonic pathway. Some of the terpenoids are highly volatile and serve as info chemicals to communicate with other plant species, herbivores, pollinators, etc. [3]. They have hypertensive, antimicrobial and insecticidal activity for which they are used as fungicides and pesticides in agriculture [4]. Similarly, essential oil extracted from different plant parts also possess some medicinal values such as antibacterial, antifungal, antiviral and nerve stimulating effect. Due to these properties essential oils are very popular in aromatherapy [5]. The volatile aromatic compounds present in these oils are used as flavoring agent in food industries, as fragrant in perfumes, aftershaves and as active component in pharmaceuticals [6, 7].
Another group of secondary metabolites are alkaloids which are basically nitrogenous compounds synthesized by different biosynthetic pathways. These compounds have been exploited as pharmaceuticals, stimulants, narcotics and poisons. The largest group of secondary metabolites from plant source is phenolic compounds having antioxidant, anti-carcinogenic alongwith other biological activity. Structurally they are diverse, commonly having hydroxylated aromatic rings but most of them are polymerized into larger compounds. In food plants, they may occur as esters or glycosides in conjugation with other natural compounds. The classification of phenolic compounds can be based on number of hydroxylic groups, chemical composition or substitutes of carbon skeleton. Phenols containing more than one hydroxylic groups are called polyphenols which are further divided into two groups- flavonoids, non-flavonoids or tannins. Both flavonoids and non-flavonoids have biological activities [8].
With the advent of modern techniques such as chromatography, electrophoresis, isotope technology and enzymology, identification of the active components, their biosynthetic pathways and mode of action is possible. In this article, secondary metabolites of the silkworm primary host plants and their medicinal properties have been briefly elucidated.
2.1. Mulberry Silkworm Host Plants
Bombyx mori solely depends on mulberry plants for food source. Generally mulberry species are found in Asia (China, Japan, and India), Southern Europe, Middle East and Northern Africa. Earlier studies have reported that mulberry leaves comprise of proteins, carbohydrates, calcium, iron, ascorbic acid, Beta carotene, vitamin B1, D and folic acid. Flavonoids such as rutin, quercitin and isoquercitin found in these plants might be responsible for its antioxidant activity [9-11]. These plants have many other therapeutic properties out of which some are shown in Figure 2.
In China, mulberry root bark is used as antiphlogistic, diuretic, expectorant; and in Japan the same herbal medicine is known as “Sohakuhi” [12]. From the UV irradiated mulberry plants, two flavonoids namely chalcomoracin and moracin were identified through HPLC. However, the former was earlier detected in the Fusarium solani infected mulberry root and stem bark. Choloromoracin has been reported with antibacterial activity against Fusarium roseun, Biopolaris leersiae, Staphylococcus aureus, methicillin resistant Staphylococcus aureus, vancomycin-resistant enterococci; antifungal activity against C. neoformans and C. albicans; and antiviral activity against rhinovirus. It also exhibits moderate cytotoxic activity against human cancer cell lines [13].
Mulberry leaf extract has antihyperglycemic, antihyperuricemic and antioxidant activity in vivo whereas xanthine oxidase inhibition and free radical scavenging activity in vitro. Additionally the extract also inhibits lipid peroxidation and glucose intake by adipocytes under the influence of insulin [14]. Another study has reported that mulberry juice when centrifuged, the supernatant had in vivo anti-stress activity and the pellet that mostly contained fibres had anti-HIV activity in Human T-cell leukemia virus I (HTLV-I)-bearing CD4-positive human T cell line [15].
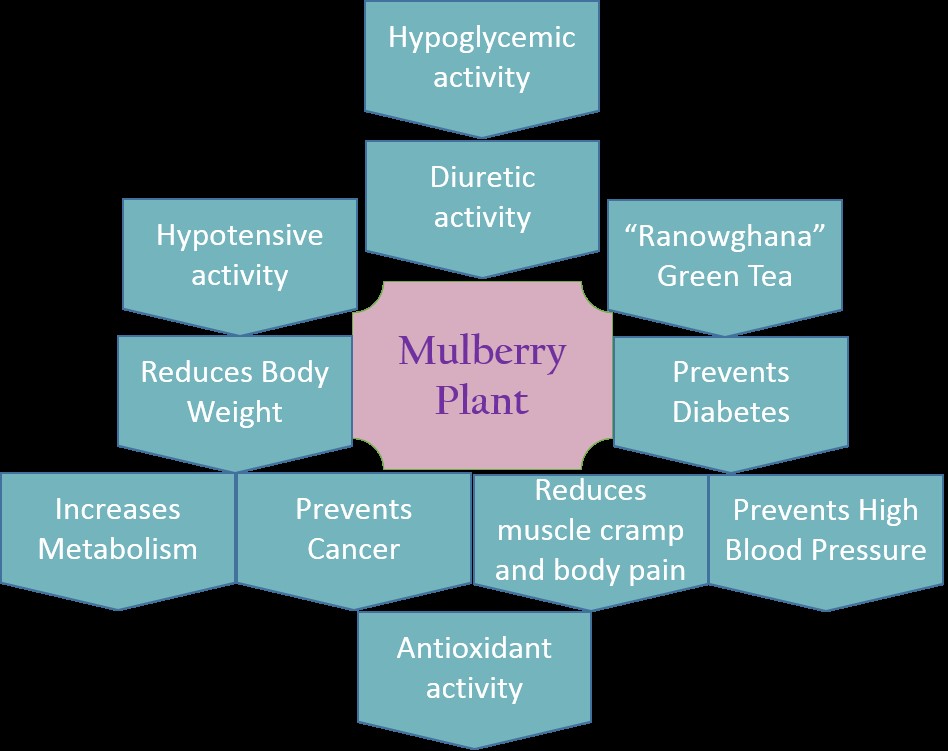
Figure 2. Medicinal properties of mulberry plants
2.2. Muga Silkworm Host Plants
Persea bombycina, Litsea monopetala and L. salicifolia are the primary host plants of Antheraea assamensis. P. bombycina trees are found in the Assam Valley of India, Khasi and Jaintia Hills of India. Studies on phenolic compounds from P. bombycina leaves at different maturity level have been carried out using HPLC. It was found that the total phenolic content decreased with the increasing maturity of the leaves. The phenolic content was also dependent on the quality of plants. Similar trend follows with β-sitosterol which is predicted to be one of the reasons for which the silkworms were more attracted towards the tender leaves of B. mori.
Chlorogenic acid which is indispensable in the silkworm synthetic diet was higher in the medium leaves. Another phenolic compound, phytic acid is actually considered as anti-nutritional factor due to their interference in proteolytic digestion and ion (Ca+2, Fe+2) absorption. The phytic acid content in P. bombycina leaves increases with the maturity of leaf. Similarly, the acid detergent lignin and fiber content was higher in the matured leaves as compared to tender and medium leaves. Non-nitrogenous phenolic tannin was found to be more in the tender leaves. It was little ambiguous that although tannin confers resistance to the seeds from pathogen attack by interfering with the food digestion, silkworms are attracted more towards the tender leaves.
The essential oil extracted from different P. bombycina varieties by hydrodstillaton method is majorly composed of aldehydes followed by acids, alcohols, dodecanal, decanal, 11-dodecanal and undecanal at different concentrations. Other minor compounds include oxides (Caryophyllene oxide, humulene 1.2-epoxide), ketone (2-tridecanone) and terpene (α-pinene) [16-17].
2.3. Eri silkworm Host plants
Eri silkworm is polyphagous in nature that feeds on 30 different host plants such as Castor, Tapoica, Wild castor, Papaya, Kesseru, etc. However, the primary one is Castor (Ricinus communis) [18]. R. communis is originally from India and Africa; however it has spread to Mediterranean countries. Castor oil has various applications for which it is commercially important. The essential oil extracted from castor leaves is used in food, drug and perfumery industries [19]. It has antimicrobial activity as it inhibits mitochondrial respiratory chain thereby curing many ailments [20].
It has been reported that the leaves, roots, seeds are used to cure inflammation, liver disorders, hypoglycemic, constipation, etc. [21-23]. Castor seed oil can be used as laxative when administered at adequate amount. People also use it to cure warts, cold tumors, in duration of mammary glands, corns and moles. Essential oil from the fresh aerial part of castor plant collected from south of Tunisia was extracted by hydro-distillation method [24-26]. In earlier studies, it was reported that R. communis essential oil has moderate antioxidant value. The GC-MS analysis of the essential oil has showed five compounds namely α-thujone, 1, 8-cineole, α-pinene, camphor, camphene that were predominantly present. The essential oil is a mixture of many compounds, so attribution to a particular compound for the antimicrobial activity will be erroneous rather it can be believed that they act collectively [27-28].
2.4. Tasar Host Plants
Antheraea mylitta producing Indian tasar silk feeds on three primary host plants Terminalia tomentosa, T. arjuna, Shorea robusta. T. tomentosa mostly occur in the forests of humid regions of India including sub-himalayan tracts of North West regions of Nepal and Sikkim and all over Peninsula of South [29]. Biochemical studies on T. tomentosa indicate that the cocoon produced by A. mylitta is superior when fed on Asan (T. tomentosa) leaves. The leaves were collected from six eco-pockets of Similipal Biosphere Reserve, Odisha and their ascorbic acid content along with protein and carbohydrate were measured. Ascorbic acid content varied region wise from 1.89 mg/g to 1.39 mg/g. It was also observed that silkworm reared in the region where leaves have highest protein and ascorbic content exhibited best cocoon performance. It can be interpreted from the cocoon performance at different eco-pockets that ascorbic acid abundance enhances molting in times [30].
- tomentosa bark has several medicinal properties. In Ayurveda, it was used for the treatment of pitta, vata, ulcers, wounds, hemorrhages, fractures, bronchitis, cardiopathy, strangury, hemoptysis, dysentery, cough, verminosis, leucorrhoea, gonorrhea and burns. It is also known to have antifungal, antioxidant, anti-hyperglycemic, antidiarrheal, anti-leucorrhea activity. Chemically the bark hexane and methanol extract contained sitosterol, betulinic acid, arjunic acid, arjunolic acid, arjunetin, ellagic acid, gallic acid and oleanolic acid [31, 32]. The ethanolic extract was found to contain carbohydrates, flavonoids, triterpenoids, steroids, tannins and saponins. Further analysis of this extract led to the isolation of four compounds 4 – methyl – 4 – hydroxymethylene – 6β – (10 – methyl octanyl) cyclohexane (Arjunahomosesquiterpenol), di-n-octylphthalate, di-isobutyl phthalate and dibutylphthalate [29].
The anxiolytic and antidepressant effects of the ethanolic extract have also been studied. The anxiolytic potential was evaluated by elevated plus maze test where mice were treated with acute dose of extract (400 mg/kg and 800 mg/kg). Increased percentage of open arms compared to control but in accordance with the positive control Diazepam was observed [33].
Another test called light dark exploration test was performed to evaluate the anxiolytic activity of the extract. Mice treated with the extract (400mg/kg and 800mg/kg) showed longer duration stay under illuminated area as compared to control groups. Forced swim test and tail suspension test are widely accepted neurobehavioral models to evaluate antidepressant activity of compounds. In their study, the ethanolic extract at 800 mg/kg p.o caused substantial decrease in the span of immobility. After the above neurobehavioral test, the rodent brains were tested for oxidation stress by GSH assay and lipid peroxidation assay. Rodent treated with acute dose of extracts (400 mg/kg and 800 mg/kg) showed decreased oxidative stress compared to the control groups [33].
2.5. Oak tasar Host Plants
Antheraea proylei is the producer of this finer tasar silk in India. Oak tasar or temperate tasar silkworm feeds on oak plant species. Sub Himalayan Belt is the natural habitat of Oak plants. Oak species (Quercus incana, Q. serrata, Q. delabata, Q. himalayana, Q. semiserrata) are its primary host plants.
- incana is distributed in the Western Himalayas outspreading to eastern Nepal. Chemically Q. incana bark contains tannins (6-23%), triterpenoid (friedelin), beta sitosterols, and leuco-anthocyanidins. The leaves contain flavonoids such as quercitin, quercitn-3-galactoside [34]. Quercuside A and B has been isolated from this plant along with lupeol and ursolic acid [35].
- serrata is native to Japan, Korea and China. A study on its seedlings suggests that herbivory induces increase in condensed tannins and total phenolic content [36]. GC-MS study of the neutralized and acidic wood vinegar chloroform extract for the analysis of phenolic compounds indicated the presence of eighteen major compounds such as 4-furancarboxaldehyde, 2-methyl phenol, 2, 6-dimethoxy phenol with other compounds [37]. Quercus genus contains four standard natural compounds ascorbic acid, quercitrin, gallic acid and rutin [38].
- CONCLUSION
Most of the silkworm host plants studied here have some medicinal properties. Some of these host plants are endemic in nature and used as herbal medicines by native people. Therefore, their therapeutic value is being limited to their native land. To explore and utilize their medicinal value in other parts of the world, efforts should be made for the purification of active compounds through modern biotechnology techniques. This will help in increasing their production and thereby, formulating medicines from them. Another major concern is antimicrobial activity of these plants. Presently, pathogenic bacteria are becoming resistant towards conventional antibiotics. Exploitation of these plants for novel compounds having anti-microbial activity can be helpful to a great extent.
- REFERENCES
- Kessler, D., and Baldwin, I. T. (2007). Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. The Plant Journal, 49(5), 840-854.
- Kabera, J. N., Semana, E., Mussa, A. R., and He, X. (2014). Plant Secondary Metabolites: Biosynthesis, Classification, Function Pharmacological Properties. Journal of Pharmacy Pharmacology, 2, 377-392.
- Maffei, M. E. (2010). Sites of Synthesis, Biochemistry Functional Role of Plant volatiles. South African Journal of Botany, 76(4), 612-631.
- Kataev, V. E., Strobykina, I. Y., Reeva, O. V., Garifullin, B. F., Sharipova, R. R., Mironov, V. F., and Chestnova, R. V. (2011). Synthesis Anti-tuberculosis Activity of Derivatives of Stevia rebaudiana Glycoside Steviolbioside Diterpenoid Isosteviol Containing Hydrazone, Hydrazide, Pyridinoyl Moieties. Russian Journal of Bioorganic Chemistry, 37(4), 483-491.
- Angelucci, F. L., Silva, V. V., Dal Pizzol, C., Spir, L. G., Praes, C. E. O., and Maibach, H. (2014). Physiological Effect of Olfactory Stimuli Inhalation in Humans: An Overview. International journal of cosmetic science, 36(2), 117-123.
- Böhme, K., Barros-Velázquez, J., Calo-Mata, P., and Aubourg, S. P. (2014). Antibacterial, Antiviral Antifungal Activity of Essential oils: Mechanisms Applications. In Antimicrobial Compounds. Springer Berlin Heidelberg. pp. 51-81.
- Burt, S. (2004). Essential oils: Their Antibacterial Properties Potential Applications in Foods—A Review. International journal of food microbiology, 94(3), 223-253.
- Ziegler, J., and Facchini, P. J. (2008). Alkaloid Biosynthesis: Metabolism Trafficking. Annu. Rev. Plant Biol., 59, 735-769.
- Rajakumar S., Chikkanna and Bindroo B.B. (2014). Food and Medicinal Values in “Silkworm” And Its Host Plant “Mulberry”-Exploring New Horizons. International Journal of Food and Nutritional Sciences, 3(1), 124-130.
- Arabshahi-Delouee, S., & Urooj, A. (2007). Antioxidant Properties of Various Solvent Extracts of Mulberry (Morus indica L.) Leaves. Food Chemistry, 102(4), 1233-1240.
- Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food chemistry, 64(4), 555-559.
- Nomura, T. (1988). Phenolic Compounds of the Mulberry Tree and Related Plants. In Fortschritte der ChemieOrganischerNaturstoffe/Progress in the Chemistry of Organic Natural Products. Springer Vienna. pp. 87-201.
- Gu, X. D., Sun, M. Y., Zhang, L., Fu, H. W., Cui, L., Chen, R. Z., … & Tian, J. K. (2010). UV-B Induced Changes in the Secondary Metabolites of Morus alba L. leaves. Molecules, 15(5), 2980-2993.
- Hunyadi, A., Liktor-Busa, E., Márki, Á., Martins, A., Jedlinszki, N., Hsieh, T. J., Bathori, M., Hohmann, J. & Zupkó, I. (2013). Metabolic effects of mulberry leaves: exploring potential benefits in type 2 diabetes and hyperuricemia. Evidence-Based Complementary and Alternative Medicine, 2013, 1-10.
- Sakagami, H., Asano, K., Satoh, K., Takahashi, K., Kobayashi, M., Koga, N. & Nakamura, W. (2007). Anti-stress, anti-HIV and Vitamin C-Synergized Radical Scavenging activity of mulberry Juice fractions. In vivo, 21(3), 499-505.
- Choudhury, S.N and Vajczikova, I. (2003). Variation in essential oil composition of Persea bombycina (King ex Hook. f.) Kost and its Effect on Muga Silkworm (Antheraea assama Ww)- A New Report. Indian Journal of Chemistry, 42B, 641-647
- Neog, K., Das, A., Unni, B. G., Ahmed, G. U., & Rajan, R. K. (2011). Studies on secondary metabolites of Som (PerseabombycinaKost), a primary host plant of muga silkworm (Antheraea assamensis Helfer). International J Pharmaceutical Science and Research, 3(3), 1441-1447.
- Venu N. Munirajappa (2013). Impact of Independent Sequential Feeding Of Different Host Plants on Economic Traits of Eri Silkworm, Philosamia Ricini Hutt. International Journal of Science Nature, 4(1), 51-56.
- Zarai, Z., Chobba, I. B., Mansour, R. B., Békir, A., Gharsallah, N., and Kadri, A. (2012). Essential oil of the leaves of Ricinus communis L.: In vitro Cytotoxicity Antimicrobial Properties. Lipids in health disease, 11(1), 1-7.
- Ferraz, A. C., Angelucci, M. E. M., Da Costa, M. L., Batista, I. R., De Oliveira, B. H., and Da Cunha, C. (1999). Pharmacological Evaluation of Ricinine, A Central Nervous System Stimulant Isolated from Ricinus communis. Pharmacology Biochemistry Behavior, 63(3), 367-375.
- Kirtikar, K. R., & Basu, B. D. (1918). Indian medicinal plants. Indian Medicinal Plants. pp-
72.
- Dhar, M. L., Dhar, M. M., Dhawan, B. N., Mehrotra, B. N., and Ray, C. (1968). Screening of Indian Plants for Biological Activity: Part I. Indian journal of experimental biology, 6, 232-247.
- Capasso, F., Mascolo, N., Izzo, A. A., and Gaginella, T. S. (1994). Dissociation of Castor Oil‐Induced Diarrhea Intestinal Mucosal Injury in Rat: Effect of NG‐nitro‐L‐arginine methyl ester. British journal of pharmacology, 113(4), 1127-1130.
- Huguet-Termes, T. (2001). New World Material Medica in Spanish Renaissance Medicine: From Scholarly Reception to Practical Impact. Medical history, 45(03), 359-376.
- Willcox, M. L., and Bodeker, G. (2004). Traditional Herbal Medicines for Malaria. Bmj, 329(7475), 1156-1159.
- Kadri, A., Gharsallah, N., Damak, M., and Gdoura, R. (2011). Chemical Composition in vitro Antioxidant Properties of Essential Oil of Ricinus communis L. J Med Plants Res, 5(8), 1466-1470.
- Zarai, Z., Chobba, I. B., Mansour, R. B., Békir, A., Gharsallah, N., & Kadri, A. (2012). Essential oil of the Leaves of Ricinus communis L.: In vitro Cytotoxicity and Antimicrobial Properties. Lipids in health and disease, 11(1), 1-7.
- Ogunniyi, D. S. (2006). Castor oil: A Vital Industrial Raw Material. Bioresource technology, 97(9), 1086-1091.
- Joshi, A. B., Aswathi, M., & Bhobe, M. (2013). Terminalia tomentosa Roxb (Ex Dc) Wight & Arn: Phytochemical Investigation. American Journal of Advanced Drug Delivery, 1(3), 224-231.
- Hota, D., Patra, G., Satapathy, P., & Satapathy, S. (2014). Biochemical Study on The Host Plant “Asan” (Terminalia tomentosa) Leaves of Tasar Silkworm Antheraea mylitta. D Collected from Eco-pockets of Similipal Biosphere Reserve, Mayurbhanj, Odisha. The Bioscan, 9(2), 609-611.
- Joshi, A. B., Bhobe, M., & Babu, A. (2013). Physicochemical and Phytochemical Investigation of Stem Bark of Terminalia tomentosa roxb (ex dc) Wight and Arn. IJAPBC, 2(3), 542-548.
- MallavarapuS, G.R., Rao, S.B., and Syamasundar, K.V. (1986). Chemical Constituents of The Bark Of Terminalia Alata. Journal of Natural Products, 49(3), 549-550.
- Das, A., & kumar Mukhopadhayay, S. (2013). Anxiolytic, Antidepressant and In Vivo Antioxidant Activity of TheEthanolic Extract of Stem Bark of Terminalia tomentosa Roxb. Indo American Journal of Pharmaceutical Research, 3(10), 8037-8045.
- Khare, C.P., Indian Herbal Remedies: Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany. 1st Edn., Springer, New York: 2004, pp. 93-94.
- Iftikhar, B., Perveen, S., Malik, A., Sultana, N., Arayne,S. and Muhammad P. (2009). Structural Determination of Quercusides A and B, New Flavonoid Glucosides from Quercus incana, by 1D and 2D NMR spectroscopy. Magnetic Resonance in Chemistry, 47(7), 605-608.
- Mizumachi, E., Mori, A. S., Akiyama, R., Tokuchi, N., & Osawa, N. (2012). Variation in Herbivory-Induced Responses within Successively Flushing Quercus serrata Seedlings under Different Nutrient Conditions. Journal of forest research, 17(2), 175-183.
- Velmurugan, N., Han, S. S., & Lee, Y. S. (2009). Antifungal Activity of Neutralized Wood Vinegar with Water Extracts of Pinus densiflora and Quercus serrata saw dusts. Int. J. Environ. Res., 3(2), 167-176
- Jamil, M., UlHaq, I., Mirza, B., & Qayyum, M. (2012). Isolation of Antibacterial Compounds from Quercusdilatata L. through Bioassay Guided Fractionation. Annals of clinical microbiology and antimicrobials, 11(1), 1-11.
