Vrunal V.More1*, Prajakta K.Khule1, Manoj M.Nitalikar2, Ritu M.Gilhotra3
- Department of Pharmaceutics, SVERI’s College of Pharmacy, Pandharpur
- Department of Pharmaceutics, Rajarambapu College of Pharmacy, Kasegaon, Sangli.
- Department of Pharmaceutics, Suresh Gyan Vihar University, Jaipur.
ABSTRACT
From the reported epidemiological studies it has been observed that the psoriasis is more prevalent in adult mostly from North India i.e. 0.44 to 2.8% whereas the prevalence of the disease in the children is found very low. The age of peak prevalence in adult is 3rd or 4th decade of their life and it is found to have male preponderance. A large population based epidemiological study for the prevalence of psoriasis is recommended to estimate the real burden of the disease in general population. The most common type of psoriasis that accounts for more than 90% cases is chronic plaque type psoriasis. The other types of morphological classification of psoriasis found in common are palmoplantar, pustular, and recalcitrant psoriasis. Psoriasis has been classified on the basis of morphological characters with an extension to widespread in localized tissue. As per the significance of clinical trial Psoriasis if classified as the Mild, Moderate and Severe. Patients with plaque type of psoriasis is prominent and likely to develop the psoriatic arthritis and the prevalence of is found to be 7-48%. A detailed history of the presence of Psoriasis is by radiological investigation is strongly recommended. However there is no consistent evidence of association of psoriasis with difference cardiovascular disease. From the available studies it is a careful to estimate the involvement of metabolic syndrome, dyslipidemia and obesity in the patients with severe psoriasis. From the current available studies it is evident the region severity index of psoriasis seems to be the most accurate and consistent diagnostic value can be utilized for the management of adult patients with plaque-type of psoriasis. Based on the latest data, the severity index for the region of psoriasis tends to be the most accurate and valid medical condition score for patients diagnosed plaque form psoriasis.
Key words: Co-morbidity, etiology, pathogenesis, psoriatic arthritis, ranking for severity.
INTRODUCTION
Psoriasis is a serious skin disorder affecting 2-3 percent of the world’s population. It can be defined as inflammatory skin condition characterized by abnormal differentiation and proliferation. It is an immune disease in which environmental and genetic factors plays very important role. Psoriasis name is derived from Greek word ‘psora’ which means ‘itch’. As discussed earlier it is inflammatory, dry, non –contagious disease which may covers entire system of person. It can be identified by scaly mariginated erythematous plaques develop on skin with symmetrical manner. Psoriasis affects on common sites such as scalp, fingers tips, plams, soles, toes, genitals, under breast, elbows, knees, shins having chances of relapse after some interval. The disease is commonly observed as white and red hues of scaly patches appearing on first layer i.e. epidermis of skin. This kind of psoriasis is known as plaque psoriasis. In some cases there are no dermatological signs and symptoms. [1]
Pathogenesis of disease
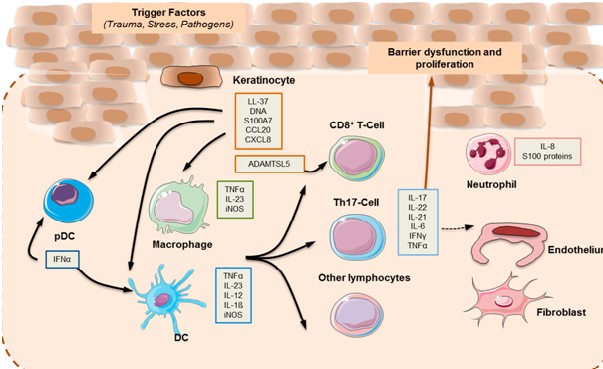
Any changes in the immune response in the innate and adaptive cutaneous tissue are responsible for the development of inflammation associated with psoriasis.[2-3] In some patients; Cytokines and activation of innate immune system by endogenous danger signal; coexist with an auto inflammatory perpetuationsand T cell-driven autoimmune reactions in others.
Hence it can be said that Psoriasis shows the traits of an autoimmune disease with an inflammatory background[4] with both mechanism of overlapping and even reciprocating one another. Clinical finding of psoriasis are morphological changes in the outermost layer of skin, which is made up of keratinocytes. The development of plaque psoriasis is not confined with inflammation of epidermal layer but extended by the interaction of keratinocytes with many different cells types like innate and adaptive immune cells, vasculature. The pathophysiology of psoriasis can be conceptualized into an initiation phase possibly initiated by trauma, infection r drugs and a maintenance phase characterized by a chronic clinical advancement.
It is notable that dendritic cells assume a significant job in the underlying phases of ailment. Dendritic cells are proficient antigen-introducing cells. Be that as it may, their enactment in psoriasis isn’t altogether clear. One of the proposed systems includes the acknowledgment of antimicrobial peptides (AMPs), which are discharged by keratinocytes because of injury and are typically over communicated in psoriatic skin. Among the most considered psoriasis-related AMPs are LL37, defensins, and S100 proteins. LL37 or cathelicidin has been credited a pathogenic job in psoriasis. It is discharged by harmed keratinocytes, and along these lines structures buildings with self-hereditary material from other harmed cells. LL37 bound to DNA animates cost like receptor (TLR) 9 in Plasmacytoid dendritic cells (pDCs).The actuation of pDC is key in beginning the improvement of the psoriatic plaque, and is portrayed by the creation of type I (IFN-_ and IFN-_). Type I IFN flagging advances myeloid dendritic cells (mDC) phenotypic development, and has been ensnared in Th1 and Th17 separation and capacity, including IFN-and interleukin (IL)- 17 creation, separately .While LL37–DNA edifices animate pDCs through TLR9, LL37 bound to RNA invigorates pDCs through TLR7. Furthermore, LL37–RNA edifices follow up on mDCs by means of TLR8 [5-6]. Enacted mDCs relocate into depleting lymph hubs and discharge tumor rot factor (TNF)- _, IL-23, and IL-12, with the last two tweaking the separation and expansion of Th17 and Th1 cell subsets, individually. Moreover, slan+ monocytes, which are significant master fiery cells found in psoriasis skin sores, react to LL37–RNA initiation by discharging high measures of TNF-_, IL-12, and IL-23 [7]. The enactment of the versatile safe reaction by means of the unmistakable T cell subsets drives the upkeep period of psoriatic aggravation [8]. Th17 cytokines, in particular IL-17, IL-21, and IL-22 actuate keratinocyte expansion in the epidermis. The provocative milieu actuates keratinocyte expansion by means of TNF-_, IL-17, and IFN-.Keratinocytes are likewise enacted by LL37 and DNA, and enormously increment the creation of type I IFNs [57]. Moreover, they take part effectively in the incendiary course through cytokine (IL-1, IL-6, and TNF-_), chemokine, and AMP emission. A broadly utilized psoriasis-like aggravation mouse model depends on the impact of the TLR7/8 agonist imiquimod, and is subsequently on the side of the TLR7/8 infection inception model. Moreover, the reaction to imiquimod was obstructed in mice lacking of IL-23 or IL-17R, which features the association of the IL-23/IL-17 hub in skin irritation and psoriasis-like pathology. The TNF_–IL-23–Th17 fiery pathway portrays plaque-type psoriasis.The IL-17 cytokine family is made out of six individuals: IL-17A–F. They are created by various cell types, and are significant controllers of incendiary reactions. Up until now, the clinically pertinent motioning in psoriasis is intervened for the most part by IL-17A and IL-17F; both act through a similar receptor, yet have various potencies. IL-17A applies a more grounded impact than IL-17F, and the IL-17A/IL-17F heterodimer has a middle of the road impact. IL-17A ties to its trimeric receptor complex made out of two IL-17RA subunits and one IL-17RC subunit, bringing about the enlistment of the ACT1 connector protein. The connection among ACT1 and the IL-17 receptor complex prompts the initiation of a progression of intracellular kinases including: extracellular sign directed kinase (ERK), p38 MAPK, TGF-beta-enacted Kinase 1 (TAK1), I-kappa B kinase (IKK), and glycogen synthase kinase 3 beta (GSK-3 beta).These kinases empower NF_B, AP-1, and C/EBP interpretation of star provocative cytokines, chemokines, and antimicrobial peptides. Th1 and Th2 cytokines act through Janus kinase (JAK)- STAT flagging pathways, though Th17 reactions are interceded by ACT1 and NF_B. On the other hand, _ T cells can create IL-17A autonomously of the IL-23 boost[9]. Medications focusing on TNF_, IL-23, and IL 17 and flagging pathways, for example, JAK/STAT are viable in the clinical administration of plaque psoriasis. Be that as it may, interchange incendiary pathways might be substantial for particular psoriatic variations.
Epidemiology
Psoriasis occurs at any age and in both sexes. Commonly psoriasis affects at age between 15-25 years. Amongst total affected 25 % have severe of moderate psoriasis. About one third of disease carring personal reports the family history hence identified with genetic loci related conditions. A genetic transmission is 25 %, but it cannot spread by person to person by contact. The numbers of cases of psoriasis are more in females than males. Children’s are rarely affected. Psoriasis can affects to all races, on the basis of rationality whites are more affected. Age 20 is more prone for onset the disease.
The exact cause behind psoriasis is not clear. But generally it is related to genetic component of an individual. In psoriasis it is observed that factors like immune systems and biochemical secretions impaired. This changes causes inflammation and proliferation of skin cells. Some probable causes other than genetic component are stress, excessive alcoholism, obesity and smoking. Pathophysiology:- Actual pathophysiology of disease is due to faulty signals from immune systems growth of skin cells is affected maturity shading time of skin cells from surface of skin is reduced to 3-6 days from 28-30 days, which starts skin cells pile up and producing lesions. Diagnosis of psoriasis can be done by observation of skin and by clinical examinations. A broad spectrum antipsoriatic treatments are available both systemic and topical for management of psoriasis while mild diseases is generally treated by topical agents, for patients with higher percentage body surface area affected both the treatments are simultaneously used to treat the disease. Treatment with systemic immunosuppressive agent can cause undesirable side effects so topical treatment of immunosuppressive agent is preferred.[10]
Classification of Psoriasis [11-16]
A. Categorisation as epidemiological
Type I psoriasis or psoriasis in the initiation phase tends to occur at or around age 40, constituting approximately 75 percent of cases. Patients are younger, have a successively intense course and also have a strong family history.The predominant part of the patients is HLA‐Cw*0602 positive.
Type II psoriasis or late-onset psoriasis occurs after age of 40, with a relevant highest at 55-60 years of age. Patients are older and also have increasingly stable psoriasis without family history. Nail association is extremely significant for HLA‐Cw*0602 negative patients in this range.
B. On the basis of morphology, size of lesion and extent
Stable/unstable
Thin (<0.75 mm)/thick (>0.75 mm)
Small plaque (<3 cm dia)/large plaque (>3 cm dia)
Localized – Flexural/intertriginous, facial/seborrheic, scalp psoriasis, palmoplantar psoriasis (nonpustular), psoriasis on limbs and trunk
Widespread – Guttate, generalized pustular, erythrodermic psoriasis
Less common varieties – Nail psoriasis, follicular psoriasis, hyperkeratotic psoriasis, photosensitive psoriasis, verrucous psoriasis
C. On the basis of age of onset, course of disease, extent of lesions, and associations
Type I – late onset of illness, not many sores including fundamentally the scalp and elbows, without related joint inflammation, atopy or affectability to natural factors, a ceaseless advancement and without familial psoriasis
Type II – Late emerging illness defined by palmoplantar psoriasis paying little attention to the closeness of the pustules, visiting joint pain and is more in smoker, fewer impacted by environmental change, but usual skin irritation.
Type III – Starting phase of disease, so much in females, few psoriasis particularize plaques mainly form in the scalp and elbow, chronic stiffness and hyperkeratosis are normal, ppt factors like stress, koebner’s or even infrequent range, linked contact dermatitis and family medical history of ordinary psoriasis are visits
Type IV – Extreme framework along with all body locations, apart from soles, forearms and nails, not linked to the time of initiation, guttate and combined injuries, less incessant to psoriatic marvel, pruritus, atopy, individual and family background of distress and smoking.
Type V- Advanced onset of chronic inflammation, less on the palms and feet, severe guttate and mixed lesions, arthritis, causes of precipitation, atopy, pruritus, psoriasis in the family and more in smokers and alcoholics.
Type VI–Described by skin lesions that cover certain locations of the body such as sole and palm, strongly associated with metabolic syndrome, extreme pustular exacerbation, mixed lesions, pruritus, allergic response, smoking but less frequent atopy.
D. On The Basis of Disease Severity
National Psoriasis Foundation
Mild psoriasis can be defined as a condition generally involving < 5 per cent of the surface area of the body. Disease does not change the quality of life of the patient (QOL), so there are no known significant risks in the therapies
Moderate psoriasis normally includes 2-20 per cent of the surface area of the body. Furthermore, the illness affects the QOL of the patient and the treatments used bear small risks.
Severe psoriasis normally includes > 10 per cent of the surface area of the body. Condition affects the Quality of life of the person does not adequately react to lower-risk medications and patients may withstand life-changing side effects in order to receive minimal or no illness. The British Association of Dermatologists defines severe psoriasis with PASI Score <3, mild 3-10 illness and DLQI < 10 sickness.
E. IADVL SIG Psoriasis:
Moderate illness (PASI / BSA < 10), moderate to extreme (PASI / BSA > 10). Patients with limited involvement in regions however with serious physical impairment or psycho – social morbidity such as severe palmoplantar psoriasis can also eligible for systematic therapy / phototherapy. One per cent of BSA is nearly equivalent to the patient’s outstretched arm (from the cuff to the fingers) with both the fingers folded together and thumbs tucked to both the sides, as indicated in the Koo-Menter Psoriasis Instrument.[34] Severe illness is often characterized in clinical trials as impacting over 10 per cent of BSA, and the US FDA used 20 per cent of BSA to recommend serious medical condition.
F. Pustular psoriasis classification
| Localized pustular psoriasis | Generalized pustular psoriasis |
| 1. palmoplantarpustulosion | 1. acute |
| 2. acrodermatitis continua | 2. From conception |
| 3. Infants and adolescents | |
| 4. circinate | |
| 5. Localised (not the feet and hands) |
Treatments for Psoriasis [17]
This will depend on the type of psoriasis that you have, and on its severity.
- Topical therapies:
Treatments that are applied directly to the skin are known as topical therapies. They include creams, ointments, pastes and lotions. If your psoriasis is mild, topical therapies will be the mainstay of your treatment. Topical therapies are discussed in more depth in another of our leaflets (Topical psoriasis remedies), which include the following:
Emollients. Emollients reduce scaling and can be used as often as needed.
Salicylic acid. Preparations containing salicylic acid can help heavily scaled plaques.
Topical steroids. Weaker steroids also do not perform very well on dense areas of psoriasis, but on the face or in the folds of skin could do well. The stronger ones have possible side effects, one of which is to make your skin thinner. Your doctor will monitor their use closely. Psoriasis sometimes comes back quickly when topical steroid treatment stops.
Tar preparations. Taking a medicated tar bath may help to remove loose scales. Tar creams or ointments help most patients but may be messy and can stain clothing.
DithranolThis can be used at home for minor or moderate psoriasis. Patients may be also treated in specialized units in hospitals. Dithranol may be effective on patients with thick plaque psoriasis; however, it is rarely used nowadays, since it may irritate the skin and also it stains not only the skin and clothing, but baths and showers.
Vitamin D analogues. There are several vitamin D preparations used to treat psoriasis; calcipotriol, calcitriol and tacalcitol. They are safe, clean to use and do not stain the skin. Treatment is applied either once (tacalcitol) or twice (calcipotriol and calcitriol) daily and can go on for as long as required. Irritation may occur, especially on the face, buttocks and genitals, and these treatments should be applied to those areas only on the specific instructions of your doctor.
Vitamin A analogues. Tazarotene is a vitamin A gel which is added to psoriasis patches once a day. Irritation can occur when added to the folds of the face or of the head. It is important to tell your doctor if you are pregnant or breast-feeding. You will stop becoming pregnant while you are being cared.
Topical immunosuppressant agents, also called calcineurin inhibitors, Tacrolimus and Pimecrolimus, are creams or ointments that are used mainly for eczema but can be effective and safe on treating psoriasis on special areas like face, folds or genitals.
Topical treatments for special sites:
Skin folds and the face. A weak steroid, a vitamin D derivative or topical immunosuppressant creams or ointments, or a tar preparation, may be prescribed for use once or twice a day. These can also be combined to increase efficacy. Regular review by your doctor will ensure that the quantities used stay within safe limits.
The scalp. A medicated tar or coconut oil shampoo may be used in addition to a steroid or calcipotriol scalp lotion. Tar or coconut oil preparations should be rubbed thoroughly into the scalp at night and washed out next morning with a tar shampoo. Wearing a shower cap overnight helps the treatment to penetrate and protects your pillowcase from stains.
The nails. There is no reliably effective treatment. Nails should be trimmed to prevent them catching.
- Phototherapy [18]
This term refers to treatment with various forms of ultraviolet light, sometimes assisted by taking particular tablets. It is helpful if the psoriasis is extensive, or fails to clear with topical treatment, or comes back quickly after seeming to clear. Topical therapy will usually continue during the phototherapy.
Two types of ultraviolet (UV) light may be given, using special machines: UVA and UVB. These are different parts of normal sunlight. Treatment with UVA is helped by taking a medication known as a psoralen – a combination known as PUVA therapy. Treatment with UVB does not need tablets
Both UVB and PUVA treatments have to be given with great care, and you will have to come up to the skin department 2 or 3 times a week for a number of weeks. Full details are given in other leaflets issued by the British Association of Dermatologists (Treatments for moderate and severe psoriasis and Phototherapy).
- Internal Treatments [19]
In cases, where the disease is very extensive or severe, patients may need oral treatment; however all of the different tablets have potential risks. In addition, you will usually have to continue with some topical therapy even though you are taking the tablets.
Your dermatologist will discuss the risks with you if you start on this kind of treatment. All of the tablets will require blood tests, and many interfere with other medicines. Female patients should not become pregnant whilst on any tablets for psoriasis, and with some of them it is important that male patients should not father a child.
Oral medications involve treatment by acitretin (vitamin A-related), ciclosporin (weakens the immune system), methotrexate (tends to slow the pace at which skin cells split into psoriasis), and hydroxycarbamide (formerly called hydroxyurea-also slows the rate at which the skin cells divide).
Several injectable forms of therapy for severe form of psoriasis are also available. Biological drugs, which target more complex defense system components, include adalimumab, ustekinumab, etanercept, and infliximab.Clear description of these therapies can be given in the patient information leaflet Therapies for moderate to serious psoriasis.
CONCLUSION
The evolution of a psoriatic lesion is based on a complex interplay between environmental and genetic factors that sets the scene for disease initiating events. A cascade of events leads to activation of dendritic cells and, in turn, the generation of effectors T cells that emigrates to and resides in skin tissue. Cross-talk between epithelial cells and immune cells shapes and maintains the inflammatory milieu. Research in the past decade has identified many of the checkpoints governing these processes and has lead to the development of new, highly effective targeted therapies. Although this progress is remarkable, there are still many unknowns, especially in the area of disease prevention and the development of drugs with appropriate long-term risk–benefit and cost profiles. Future research will need to tackle these challenges in order to establish therapeutic and preventive approaches that ultimately lead to improved outcomes for patients.
REFERENCES
- Sampogna, F.; Tabolli, S.; Abeni, D. Living with psoriasis: Prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm. Venereol. 2012, 92, 299–303.
- Di Meglio, P.; Villanova, F.; Nestle, F.O. Psoriasis. Cold Spring Harb. Perspect. Med. 2014, 4, 6.
- Harden, J.L.; Krueger, J.G.; Bowcock, A.M. The immunogenetics of psoriasis: A comprehensive review. J. Autoimmun. 2015, 64, 66–73.
- Liang, Y.; Sarkar, M.K.; Tsoi, L.C.; Gudjonsson, J.E. Psoriasis: A mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 2017, 49, 1–8.
- Morizane, S.; Gallo, R.L. Antimicrobial peptides in the pathogenesis of psoriasis. J. Dermatol. 2012, 39, 225–230.
- Morizane, S.; Yamasaki, K.; Muhleisen, B.; Kotol, P.F.; Murakami, M.; Aoyama, Y.; Iwatsuki, K.; Hata, T.; Gallo, R.L. Cathelicidin antimicrobial peptide ll-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Investig. Dermatol. 2012, 132, 135–143.
- Nestle, F.O.; Conrad, C.; Tun-Kyi, A.; Homey, B.; Gombert, M.; Boyman, O.; Burg, G.; Liu, Y.J.; Gilliet, M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005, 202, 135–143.
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015, 43, 727–738.
- Hansel, A.; Gunther, C.; Ingwersen, J.; Starke, J.; Schmitz, M.; Bachmann, M.; Meurer, M.; Rieber, E.P.; Schakel, K. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J. Allergy Clin. Immunol. 2011, 127, 787–794.
- Van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845.
- Guinot C, Latreille J, Perrussel M, Doss N, Dubertret L; French Psoriasis Group. Psoriasis: Characterization of six different clinical phenotypes. Exp Dermatol 2009;18:7129.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55,78.
- Chandran V, Schentag CT, Gladman DD. Sensitivity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum 2007;57:1560,3.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P; CASPAR study group. Classification criteria for psoriatic arthritis. Arthritis rheum 2006;54:2665,
- Griffiths CE, Christophers E, Barker JN, Chalmers RJ, Chimenti S, Krueger GG, et al. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 2007;156:258,62.
- Pariser DM, Bagel J, Gelfand JM, Korman NJ, Ritchlin CT, Strober BE, et al. National Psoriasis Foundation Clinical Consensus on Disease Severity. Arch Dermatol 2007;143:239,24.
- Feldman SR, Koo JY, Menter A, Bagel J. Decision points for the initiation of systemic treatment for psoriasis. J Am Acad Dermatol 2005;53:101,7.
- Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologist’s guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987,1019
- Gupta SK, Singh KK, Lalit M. Comparative therapeutic evaluation of different topicals and narrow band ultraviolet B therapy combined with systemic methotrexate in the treatment of palmoplantar psoriasis. Indian J Dermatol 2011;56:165‑
