pp- 1-10
Bhaskar Sharma1* and Md. Sufiyan Siddiqui1
1Department of biochemistry & Bioprocess Technology, Sam Higginbottom Institute of Agriculture, Technology & Sciences , Allahabad- 211008 (U.P) India
*Corresponding Author – reserachpaper26@gmail.com
Abstract
Diabetes mellitus is a universal problem affecting human societies at all stages of development. It is a condition where sufficient amount of insulin is either not produced or the body is unable to use the insulin that is produced, leading to excess glucose in the blood. The main aim present study was to investigate protective effect of aqueous extract against oxidative stress and renal damage in alloxan induced mice. Diabetes was induced in mice by alloxan monohydrate at dose of 150 mg/kg body weight injected intraperitoneally. Also alloxanized induced mice were administered with 300 and 500 mg/kg body weight orally daily of extract for a period of 21 days. At the end of the administration period, the mice were anaesthesized and dissect for the collection of blood and kidney tissues. In diabetic mice, the serum glucose levels as well as urea, uric acid , Creatinine level were significantly increased (p<0.001) and albumin , protein level were decreased in comparison with the control groups. Diabetic mice group treated by extract at the dose of 300 and 500 mg/kg body weight orally significantly (p<0.001) reduced and normalised these biochemical parameters compared with alloxan induced diabetic group. Histopathological study also did show adverse alternation in the morphological architecture of the kidney tissue. The results suggested that aqueous suspension of aloe vera leaves possesses protective effect against oxidative stress and renal damage in alloxan induced diabetic mice.
Key word: Alloxan, Mus musculus, KFT, LPO
Introduction
Diabetes mellitus (DM) has been defined by a persistently elevated blood glucose concentration, leading to complications that can be acute and long term [1] Globally, DM presents enormous and increasingly important public health issues. The prevalence of DM in all age groups was estimated to be 2.8% (170 million) in 2000 and the rate is expected to rise to 4.4% (366 million) in 2030 [2] The occurrence and consequences associated with diabetes are found to be high in countries like India (31.7%), China (20.8%) and USA (17.7%). The rate is expected to rise to 79.4%, 42.3% and 30.3%, respectively, by 2030 in the above countries [3]. The worldwide survey on diabetes reveals that among the entire diabetes cases more than 90% are account to type-II [4]. The overall death rate in people with diabetes is about twice that of people without diabetes [5].
Diabetic nephropathy (DN) is one of the important microvascular complications of diabetes mellitus. Recent studies indicate that reactive oxygen species (ROS) play a key intermediate role in the pathophysiology of diabetic nephropathy [6]. Hyperglycaemia, the main determinant of the initiation and progression of diabetic nephropathy not only generates more reactive oxygen metabolites but also attenuates anti-oxidative mechanisms through non-enzymatic glycosylation of anti-oxidant enzymes [7].The mechanism by which hyperglycaemia causes free radical generation and thus causes oxidative stress is complex. High glucose concentration directly increases hydrogen peroxide production by murine mesangial cells and lipid peroxidation of glomeruli and glomerular mesangial cells [8]. Hyperglycaemia promotes glycosylation of circulating and cellular protein and may initiate a series of auto-oxidative reactions that culminate in the formation and accumulation of advanced glycosylation end-products (AGE) in tissue proteins [9-10]. The AGE has oxidizing potential and can promote tissue damage by free radicals. In addition, increased lipid peroxidation impairs membrane functions by decreasing membrane fluidity and changing the activity of membrane-bound enzymes and receptors. Its products (lipid radicals and lipid peroxides) are harmful to the cells in the body and associated with atherosclerosis and damage to brain, kidney, liver and other tissue [ 11]. In addition, diabetes and hyperglycemia can be sources of DNA damage via the oxidation of DNA bases and sugar phosphate binding sites [12]. The occurrence of these alterations can result in mutagenic effects and DNA replication arrest and could be associated with risks for developing cancer in diabetes mellitus patients [13-14].
The beneficial uses of medicinal plants in traditional system of medicine of many cultures are extensively documented. Several plants have been used as dietary adjuvant and in treating the number of diseases even without any knowledge on their proper functions and constituents. This practice may be attributed to the uncompromised cost and side effects of synthetic hypoglycemic agents [15]. Since antique era, plants with medicinal properties are enormously used in treating diabetes throughout the world. Many recent scientific investigations have also confirmed the efficacy of plant preparations, few of which are remarkably effective [16]. Aloe vera is a traditional remedy for diabetes mellitus (DM) in many parts of the world, including latin america [17] and the Arabian Peninsula [18]. Some evidence in humans and animals suggests that Aloe vera is able to alleviate the chronic hyperglycemia and perturbed lipid profile that are characteristic of DM, which are major risk factors for cardiovascular complications in the disease.
Materials and methods
Experimental animals
The study was carried out in Healthy Swiss albino mice (Mus musculus) (4-6 months old ) of either sex ranging from 28 to32 g that were obtained from the Experimental animal facility of Mahavir cancer Sansthan and research centre (Patna , Bihar) after getting the approval of Institutional Animal Ethics Committee and the experiments were carried out as per the guidelines of “CPCSEA guidelines for laboratory animal facility” (Committee for the Purpose of Control and Supervision on Experiments on Animals) and the approval number is (CPCSEA Regd. No.1129/bc/07/CPCSEA, dated 13/02/2008). The mice were acclimatized for a period of one week before starting the experiments.
Collection of Plant material
The leaf of aloe vera were collected from local garden, Allahabad, U.P, India. The plant species was authenticated by botanist, Department of Botany, Sam Higginbottom Institute of Agriculture, Technology & Sciences, Allahabad- 211008, U.P, India. The leaf were shaded-dried with occasional shifting and then powdered with mechanical grinder passing through sieve and stored in air-tight container.
Preparation of extracts
After shaded drying the dried leaf were powdered in mechanical grinder. The powdered leaf was macerated with distilled water for 72 hrs at room temperature with occasional stirring. It was then filtered through filter paper, the filtrate was dried and stored in refrigerator further use. During experiment the crude extract was diluted with distilled water just before administration to animals.
Experimental design
Four groups of mice, six mice in each received the following treatment schedule.
Group I: Normal control (NC)
Group II: Diabetic control (DC) (alloxan 150 mg/kg body weight i.p)
Group III: Diabetic control (DC) (alloxan 150 mg/kg body weight i.p) + C300 [aloe vera-leaves extract at the dose of 300 mg /kg b.w).
Group IV: Diabetic control (DC) (alloxan 150 mg/kg body weight i.p) + C500 [aloe vera-leaves extract at the dose of 500 mg /kg b.w).
Chemicals
All chemicals were obtained from the following sources: alloxan was purchased from the Loba chemie, Mumbai. Commercially available kits for bio-chemical analyses such as glucose, urea, Creatinine were done using commercial diagnostic kits following manufacturer’s instructions. All reagents used in study were of analytical grade.
Induction of diabetes in experimental animals
Diabetes mellitus was induced in overnight fasted mice by a single intraperitoneal injection of alloxan monohydrates at the rate of 150 mg/kg body weight [19]. The animals were allowed free access to 5% glucose solution to overcome the drug induced hypoglycaemia. Blood glucose level of these mice were estimated 72 hr after alloxan administration, diabetes was confirmed by blood samples collected from the tip of the tail using a blood glucometer (Accu Sure , Taiwan). Animals with blood glucose level equal or more than 200 mg/ dl were declared diabetic and were used in entire experimental group [20].
Collection of blood sample and estimation of serum biochemical investigations
Blood samples were collected by orbital sinus puncture method [21]. Serum was prepared following procedure. Briefly, blood samples were withdrawn from orbital sinus using non-heparinised capillary tubes, collected in dried centrifuge tubes and allowed to clot. Serum was separated from the clot by centrifuged at 3000 rpm for 15 min. at room temperature. Serum was collected carefully and kept at -20°C until analysis of fasting blood glucose concentration was determined by commercially available glucose kit (crest coral clinical system, Goa, India) based on Trinder’s [22], Kidney function tests, including determination of urea [23] , uric acid [24] , albumin [25] , Protein[26] and Creatinine [27] in serum were done using diagnostic kits (crest coral clinical system, Goa, India). Lipid peroxidation was estimated by Ohkawa et al., [28]
Histological analysis of kidney Tissues
At the end of the experimental period, the whole kidney tissues from each animal was removed after sacrificing the animal by cervical dislocation and a portion of the kidney was cut into two to three pieces of approximately 6 mm3 sizes and fixed for 48 h in 10% formalin saline were dehydrated by passing successfully in different mixture of ethyl alcohol-water, cleaned in xylene and embedded in paraffin wax. Thin sections of 5 µm thickness of kidney tissue were cut and then stained with haematoxylin and eosin dye (H&E), which mounted in neutral deparaffinated xylene (DPX) medium for microscopic observation. The thin sections of kidney were made into permanent slides and examined [29] under high resolution microscope with photographic facility and photo micrographs were taken.
Data analysis
Data from the experiments were presented as mean ± Standard deviation. Statistical analysis was done by using the GraphPad Prism Program (GraphPad Software, Inc., San Diego, USA). Paired T-test was done to see any difference between the paired groups. The level of significance was set at p < 0.001.
Results
Effect of the aqueous suspension of aloe vera on serum glucose levels in diabetic mice
After induction of diabetes by alloxan, diabetes was confirmed by the presence of hyperglycemia in animals and the mean level of glucose in the control group of mice was evaluated to be 75.92 ± 2.82 mg/dl (range 69.09-88.98) whereas it was 228.6 ± 3.51 mg/dl (range values 215 to 240, p=0.0001, t =31.08 ) in alloxanized group. After the treatment of mice with the fruit extract of aloe vera (300 mg /kg body weight) the glucose level decreased down to 189.5 ±4.40 mg/dl (p= 0.0023, t=5.70) having a range of 170-200 mg/dl and more potent effect at the dose of 500 mg/kg body weight of extract the level of glucose also significantly decreased to 93 ± 5.62 mg/ dl (p=0.0001, t=38.10) having range of 65-101 mg/ dl. These variations in glucose concentrations are evident from Figure 1. The significant increase in glucose concentration in the diabetic animals than that of the control mice is evident on alloxanization. However, the oral administration of aqueous extract of aloe vera significantly reduced the glucose level in serum when compared with alloxan induced diabetic mice.
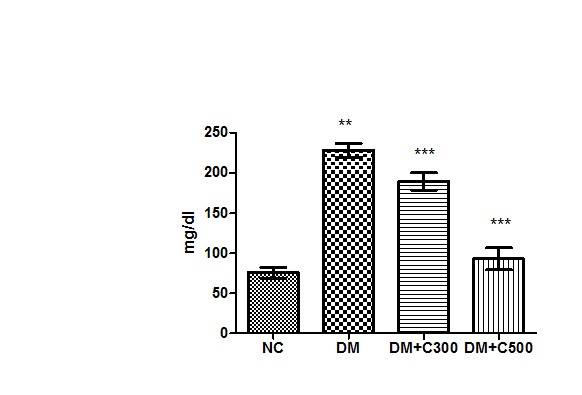
Figure1 : Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum glucose levels in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Effect of the aqueous suspension of aloe vera on serum urea levels in diabetic mice
In control group of mice urea activity was found to be 14.26 ± 0.76 mg/dl having the range of 12 to 17 mg/dl. In diabetics, its activity got raised to 25 ± 1.60 mg/dl (p= 0.0012, t=6.65) with variations range from 20 to 30. However, extract (300 and 500 mg/kg b.w) treatment of this group for three weeks resulted in decrease of urea activity to 23 ± 1.54 and 15.67± 0.71 (p= 0.2164, t=1.41 and p= 0.0017, t= 6.13) having values ranging from 19 to 30 mg/dl and 13 to 18 mg/dl. These variations are depicted by the box-plot in Figure 2.
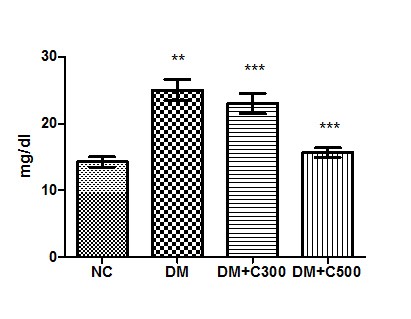
Figure2 : Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum urea levels in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Effect of the aqueous suspension of aloe vera on serum uric acid levels in diabetic mice
In control group of mice uric acid activity was found to be 4.07 ± 0.45 mg/dl having the range of 2.35 to 5.63 mg/dl. In diabetics, its activity got raised to 11.84± 0.79 mg/dl (p = 0.0001, t=11.01) with variations range from 10 to 15. However, extract (300 and 500 mg/kg b.w) treatment of this group for three weeks resulted in decrease of uric acid activity to 7.22 ± 0.66 and 5.07± 0.47 (p= 0.0086, t=4.18 and p= 0.0012, t= 6.59) having values ranging from 4.98 to 9.65 mg/dl and 3.56 to 6.98 mg/dl. The overall alterations in these groups are shown in Figure 3
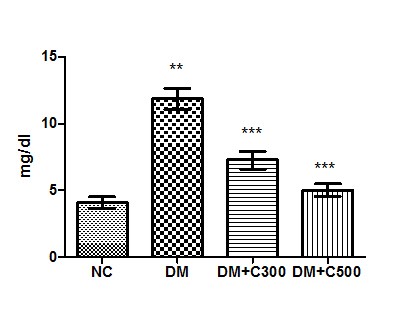
Figure3 : Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum uric acid levels in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Effect of the aqueous suspension of aloe vera on serum Creatinine levels in diabetic mice.
The estimation of serum Creatinine level in control group revealed its level to be 0.59± 0.03 mg/dl with individual level variations range from 0.47 to 0.72mg/dl. In alloxan treated diabetic group, the mean value was 1.29± 0.04 mg/ dl with individual level variations range from 1.11 to 1.45 mg/ dl whereas in extract treated at dose of 300 mg/kg b.w, the mean value was 0.91±0.03 mg/ dl of the values ranging from 0.79 to 1.01 mg/ dl and at dose of 500 mg/ kg b.w, its mean value was 0.74±0.05 mg/ dl with ranging from 0.54 to 0.90. This showed an significant increase (p=0.0001, t=12.15) when compared with control group, however, the decline in extract treated level was significant (p=0.0012, t=6.64 and p= 0.0001, t= 10.36) as compared to alloxan treated group (Fig:4)
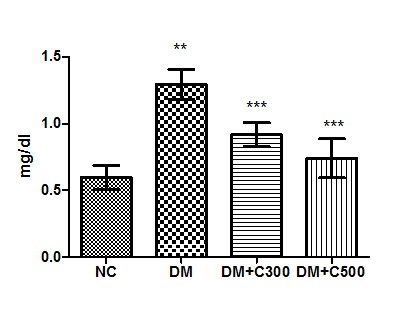
Figure4 : Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum Creatinine acid levels in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Effect of the aqueous suspension of aloe vera on serum albumin levels in diabetic mice
In control group of mice albumin activity was found to be 4.23 ± 0.40 g/dl having the range of 3.00 to 5.80 g/dl. In diabetics, its activity got declined to 2.52± 0.18 g/dl ( p= 0.0085, t=4.20) with variations range from 1.87 to 3.05. However, extract (300 and 500 mg/kg b.w) treatment of this group for three weeks resulted in increase of albumin activity to 3.45 ± 0.28 and 3.91± 0.14 (p= 0.0219, t=3.28 and p= 0.0044, t= 4.91) having values ranging from 2.35 to 4.23 g/dl and 3.45 to 4.5 g/dl. These variations along with statistical significance are depicted by box-plot as shown in fig:5.
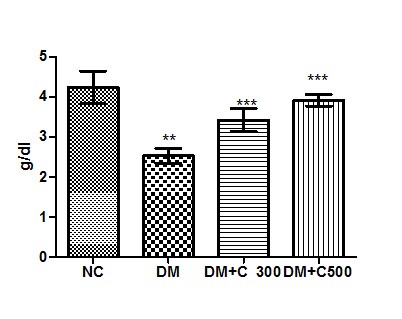
Figure5 : Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum albumin levels in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Effect of the aqueous suspension of aloe vera on serum protein levels in diabetic mice
In Control group of mice protein activity was found to be 5.33 ± 0.70 g/dl having the range of 4 to 9 g/dl. In diabetics, its activity got declined to 3.93± 025 g/dl ( p= 0.0109, t=3.94) with variations range from 3.09 to 5.00. However, extract (300 and 500 mg/kg b.w) treatment of this group for three weeks resulted in increase of protein levels to 4.62 ± 0.48 and 5.45± 0.47 (p= 0.0462, t=2.63 and p= 0.0181, t= 3.45) having values ranging from 3.090 to 6.340 g/dl and 3.85 to 7.24 g/dl. These variations along with statistical significance are depicted by box-plot as shown in fig:6.
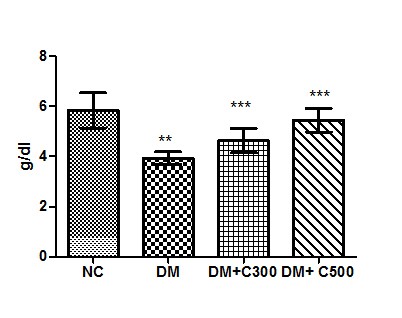
Figure6: Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum protein levels in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Effect of the aqueous suspension of aloe vera on serum lipid peroxidation activity in diabetic mice
The estimation of serum lipid peroxidation level in control group revealed its level to be 1.20± 0.05 nmole/ml with individual level variations range from 1.021 to 1.350 mg/dl. In alloxan treated diabetic group, the mean value was 2.16 ±0.06 nmole/ml with individual level variations range from 1.98 to 2.39 nmole/ml whereas in extract treated at dose of 300 mg/kg b.w, the mean value was 1.70 ±0.13 nmole/ml of the values ranging from 1.31 to 2.18 nmole/ml and at dose of 500 mg/ kg b.w, its mean value was 1.34±0.041 nmole/ml with ranging from 1.21to 1.49. This showed an significant increase (p=0.0001, t=17.15) when compared with control group, however, the decline in extract treated level was significant (p =0.0269, t=3.09 and p= 0.0001, t= 16.77) as compared to alloxan treated group (Fig:7)
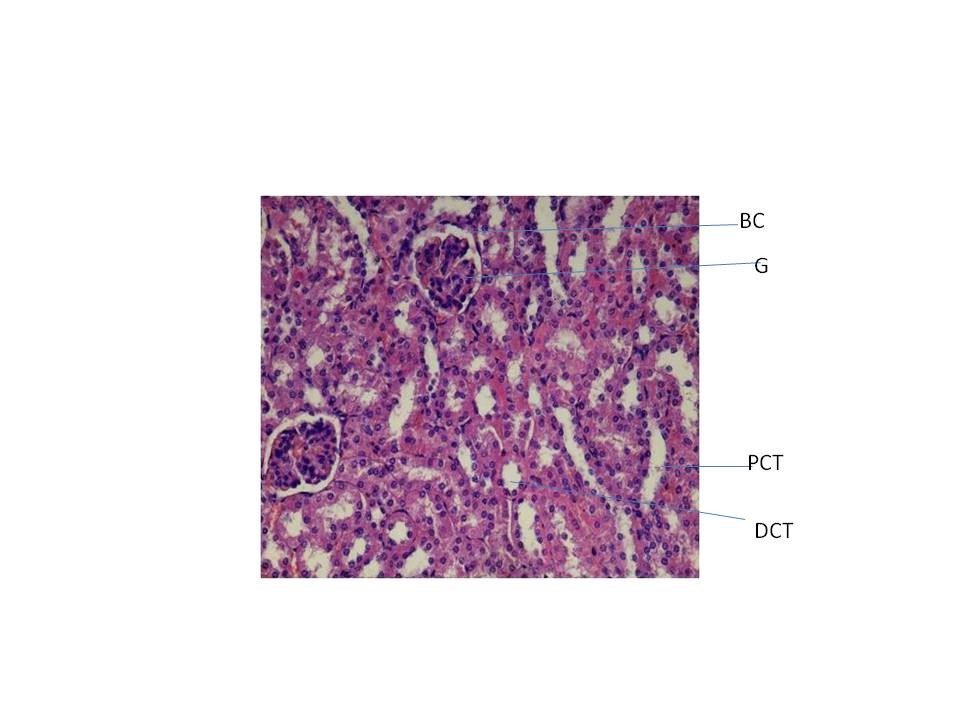
Figure7: Effect of aqueous extract of aloe vera (100 and 250 mg/ kg b.w) on the serum lipid peroxidation activity in alloxan induced mice. Values are the means ±S.E.M for six animals in each group. Values are significant at p<0.001, statistical significance was compared within groups as follows. ** Diabetic mice were compared with normal mice.***DM+C300 and DM+C500 treated diabetic mice were compared with diabetic mice.
Histopathology study
From fig : 8 showed histological study kidney of control mice showed well rejuvenated renal corpuscles (glomerulus and Bowman’s capsule) with normal proximal and distal convoluted tubules. However, induction of diabetes with alloxan was associated with marked histological changes in the kidney tissues as revealed by degeneration in proximal , distal convoluted tubules and also caused degeneration in glomerulus with increased capsular space (Fig:9). Treatment of diabetic mice with the aqueous suspension of aloe vera afforded significant protection from renal damage (Fig:10)
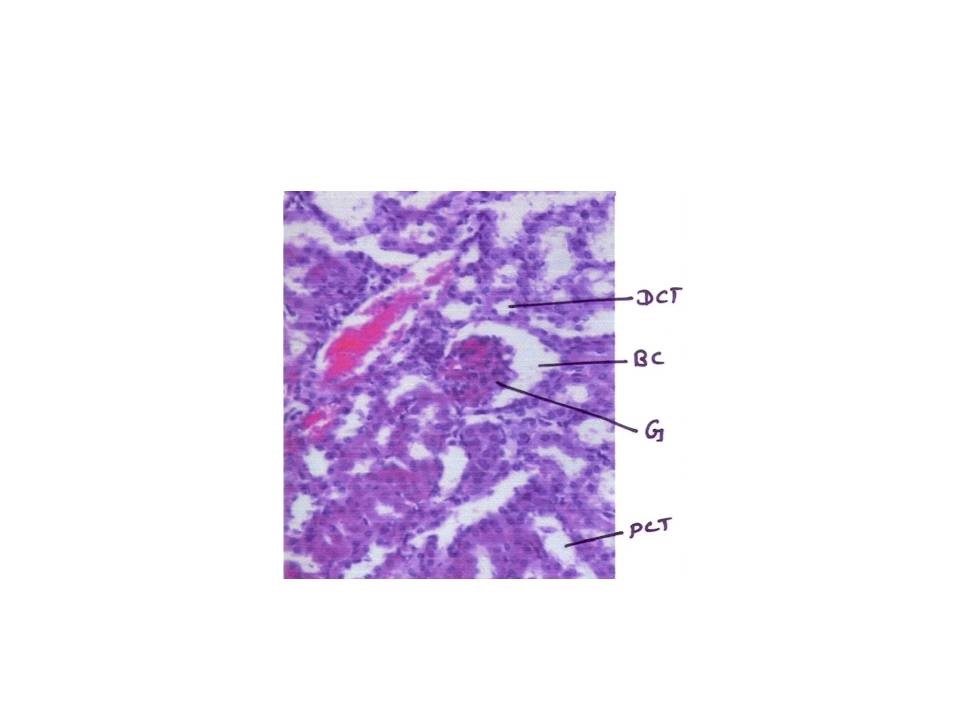
Fig:8 [Photomicrograph of control mice kidney section (day 21) showing normal histoarchitecture with well developed proximal and distal convoluted tubules (PCT, DCT) and normal glomerulus and Bowman’s capsule (G and BC) H and E X200]
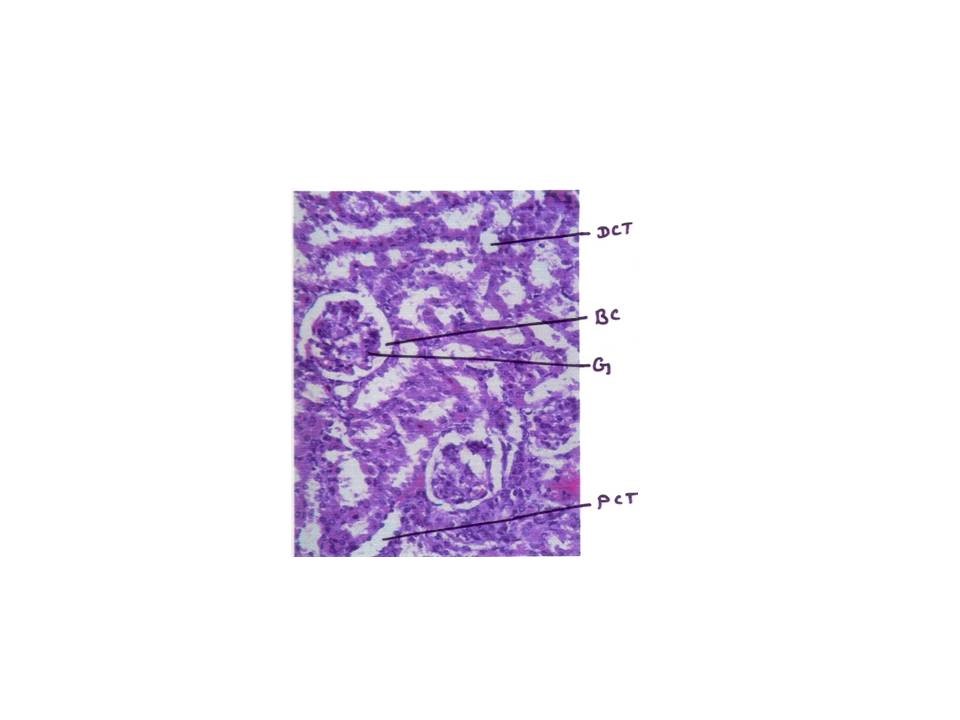
Figure: 9 [Photomicrograph of diabetic mice kidney section (day 21) showing degenerated glomerulus with distorted proximal and distal convoluted tubules, H and E X200]

Figure:10 [Photomicrograph of mice kidney section of aqueous treated showing well rejuvenated proximal and distal convoluted tubules with glomerulus and Bowman’s capsule H and E X200]
Discussion
Plants generally have varied chemical compositions depending upon species. A good number of plants are known to be of economic and medicinal value. Those that are of medicinal value are often used as herbal remedy for the restoration and maintenance of good health. Some herbs have been considered as drugs and therefore generally safe and effective. Most herbs have been associated with broad actions on a number of physiological systems in concert unlike the pharmaceutical drugs which are usually designed to elicit a specific effect. Some researchers on medicinal plants are of the opinion that some herbal plants are usually oriented in the same general therapeutic direction and are complementary or synergistic, often non-specific but very rarely adverse [30]. Medicinal plants are being used traditionally in many parts of the world in the treatment of diabetes mellitus where access to formal healthcare is limited [31] and these medicinal plants could play important roles in the lives of rural people, particularly those in remote parts of developing countries.
In view of the traditional use of aloe vera species in treating diabetes and lowering blood glucose levels in experimental conditions, the present study was carried out to evaluate the efficacy of aqueous suspension of aloe vera in affording protection against renal damage in diabetic mice and to elucidate its mechanism of action. Diabetes was induced in mice by single intraperitoneal injection of alloxan (150 mg / kg body weight), Alloxan is a β-cytotoxin, induces diabetes mellitus by damaging the insulin secreting β-cells of the pancreas, resulting in decreased endogenous insulin release. Alloxan-administered rabbits become hyperglycemic in a short period of time, followed by hepatic glucose overproduction [32]. High ambient glucose can promote apoptosis, suggested by [33] causing potential cellular damage as a result of hyperglycemia in diabetes. Reactive oxygen species (ROS) are important mediators of β-cell death during the development of DM. High glucose has been postulated to generate ROS and nitrogen species in numerous cell types. Generation of superoxide by high glucose is well described and arises principally via the mitochondrial electron transport chain [34]. Another source of glucose induced oxidative stress is via the polyol pathway where glucose is reduced to sorbitol by aldose reductase in a process that consumes NADPH. This will impair the NADPH-dependent generation of glutathione an essential cellular antioxidant [35]. Treatment of aloe vera in alloxan induced diabetic mice started reducing glucose level. The antihyperglycemic effect of aloe vera at 500 mg /kg b.w dose was found to be more effect than 300 mg/kg b.w. This results are agree with another finding [36-39].In this respect, Ayesha et al., [40] reported that there are two possible explanations for the antidiabetic property of aloe vera. It may have exerted its effect by preventing the death of β-cells and it may permit recovery of partially destroyed β-cells, aloe vera may also have initiated cell proliferation
The antidiabetic activity of aqueous extract of aloe vera may be its promote insulin secretion by closure of K+– ATP channels, membrane depolarization and stimulation of calcium influx, an initial key step in insulin secretion. In this context, number of other plants has also been reported to have antidiabetic and insulin stimulatory effects [41]. Flavonoids sterols, triterpenoids, alkaloids and phenolics are known to be bioactive antidiabetic principles [42]. Flavonoids are known to regenerate the damaged β-cells in the alloxan induced diabetic rats[43] Phenolics are found to be effective antihyperglycemic agents. Effective blood glucose control is the key for preventing or reversing diabetic complications and improving the quality of life in patients with diabetes. On this basis we have selected the glucose induced hyperglycaemic model to screen the anti-hyperglycaemic activity of the plant extracts.
Nephropathy is one of the serious complications of diabetes that is associated with the excretion of albumin in urine and is the leading cause of end-stage renal failure [44-45]. The development and progression of renal disease is indicated by the appearance of proteinuria [46]. In the current study, a single alloxan injection affected kidney functions and produced a marked increase in serum urea, uric acid and Creatinine value and decreased albumin , protein value when compared to non-diabetic group. This may be due to the protein glycation in diabetes which may lead to muscle wasting and increased release of purine. The main source of uric acid as well as increased activity of xanthine oxidase. Our results are consistent with those reported by others [47-49] who showed that serum uric acid, urea and Creatinine levels were increased in diabetic rats. This may due to metabolic disturbance in diabetes reflected in high activities of xanthine oxidase, lipid peroxidation and increased triacylglycerol and cholesterol levels. Similar results were reported [50] showing the increased concentrations of urea and Creatinine due to excessive lipolysis in severe diabetic mellitus leading to ketosis and later acidosis. Kidney maintains optimum chemical composition of body fluid by acidification of urine and removal of metabolic wastes such as urea, uric acid and Creatinine. During renal diseases the concentration of these metabolites increases in blood [51]. On the other hand treatment of extract for 21 days on diabetic mice, the elevated level were become normalize [52-54].
In this regard, the decrease of serum albumin and protein in diabetic animals was restored to control rate by insulin treatment, which accelerates amino acid transport through cells and stimulates the protein manufacturing machinery of the cell [55]. Reduction in plasma albumin was observed in alloxan induced mice which may be due to microproteinuria and albuminuria, which is an important clinical marker of diabetic nephropathy [56-58] and or may be due to increased protein catabolism [59]. Lack of insulin also reduces RNA and mRNA, which is another factor for the reduction of total protein [60]. Our results also correlate with the above findings. Lipid peroxides are the secondary products of oxidative stress and are unleashed as a result of the toxic effect of reactive oxygen species produced during lipid peroxidation in diabetes [61]. Lipid peroxidation (LPO) is one of the cellular features of chronic diabetes. In diabetes, it is thought that hypoinsulinemia increases the activity of the enzyme, fatty acyl coenzyme-A oxidase, which initiates β-oxidation of fatty acids, resulting in LPO [62]. Increased LPO impairs membrane function by decreasing membrane fluidity and changing the activity of membrane-bound enzymes and receptors [63]. LPO will in turn result in elevated production of free radicals that are harmful to cells in the body [64]. Moreover, lipid peroxide mediated tissue damage has been observed in the development of both type I and II diabetes mellitus and insulin secretion is closely associated with lipoxygenase-derived peroxides. The increased LPO leads to cellular infiltration and islet cell damage in diabetes [65]. During this study, elevated levels of lipid peroxidation were noticed in alloxan treated mice. There are several reports in the literature that demonstrated the elevated levels of lipid peroxides in the alloxan induced diabetes [66]. This normalization may be accomplished by the antioxidant and free radical quenching nature of alove vera .
Conflicts of interest
Authors declare that there is no conflict of interests regarding the publication of this paper. Acknowledgements
Authors are thankful to Director, Mahavir Cancer Sansthan & Research Centre, Patna. Bihar (India) for providing required facilities for the current study. We also thank Head of the Department for providing the animals for the present work.
Bibliography
[1] Greenbaum, Carla J., Harrison, Leonard C, “Diabetes: Translating Research into Practice”. Informa Health Care, New York, London, pp. 1–2, 2008
[2] Fonseca, Vivian A, “Clinical Diabetes: Translating Research into Practice”. Saunders – An Imprint of Elsevier, pp. 2–3 (Chapter 1). 2006
[3] W. Sarah, M.B Bchir,., Roglic, Gojka, Green, Ander, Sicree, Richard, King, Hilay, “Global prevalence of diabetes; estimates for the year of 2000 and projection for 2030”. Diabetes Care vol.27, no.5, pp.1047–1053, 2004.
[4] National Diabetes Fact sheet ,“Centers for Disease Control and Prevention”. 2005
[5] R.A , Harrigan., M.S , Nathan, P. Beatie, “Oral agents for the treatment of type 2 diabetes mellitus: pharmacology, toxicity, and treatment”. Ann. Emerg. Med. vol.38, no.1, pp. 68-78, 2001.
[7] N. Orsolic , I. Basic, “Honey Bee Products and their Polyphenolic Compounds in Treatment of Diabetes. In Phytopharmacology and Therapetutic Values IV Edited by Govil JN, Singh VK. LLC, U.S.A: ; Stadium Press, vol. 22, pp.455–471. 2008
[6] H .Ha , I.A .Hwang , J.H. Park , H.B .Lee , “Role of reactive oxygen species in the pathogenesis of diabetic nephropathy”, Diabetes Res Clin Pract. Vol.13, no.82 pp. 42-5, 2008
[8]. L.M. Ruiz-Munoz, F .Vidal-Vanaclocha, I. Lampreabe, “Enalaprilat inhibits hydrogen peroxide production by murine mesangial cells exposed to high glucose concentrations”, Nephrol Dial Transplant vol.12,no.3, pp. 456–464, 1997
[9] J.V . Hunt, M.A . Bottoms, M.J .Mitchinson. “Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production”, Biochem J vol.15, no.291, pp.529–535, 1993.
[10] M .Anjaneyulu , K .Chopra. “ Effect of irbesartan on the antioxidant defence system and nitric oxide release in diabetic rat kidney”, Am J Nephrol, vol.24, no.5, pp. 488–496, 2004
[11] K.K . Yue , S.N. Leung, P.M .Man, W.F .Yeung , W.S. Chung, K.W .Lee, A.W .Leung, C.H .Cheng. “Alterations in antioxidant enzyme activities in the eyes, aorta and kidneys of diabetic rats relevant to the onset of oxidative stress”, Life Sci, vol.77, no.7, pp.721–734, 2005
[12] N . Orsolic, G. Gajski, V .Garaj-Vrhovac, D .Dikic, Z.S .Prskalo, D .Sirovina. “DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice”, Eur J Pharmacol vol.10, no.656, pp.110–118, 2011.
[13] J.C ,Will, F .Vinicor, E.E . Calle, “Is diabetes mellitus associated with prostate cancer incidence and survival”, Epidemiology vol.10, no.3, pp.313–318, 1999
[14] P .Maisonneuve, L .Agodoa, R .Gellert, J.H .Stewart, G .Buccianti, A.B .Lowenfels, R.A.Wolfe, E .Jones, A.P .Disney, D .Briggs, M .McCredie, P .Boyle, “Cancer in patients on dialysis for end-stage renal disease: an international collaborative study”, Lancet vol.10, no.354, pp.93–99, 1999.
[15] Taylor, B. John ,Triggle, J. David. “Comprehensive Medicinal Chemistry – II. Global Perspective”, Text Book, vol. 1. Elsevier, pp. 357. 2006
[16] R.J, Marles, N.R ,Farnsworth, “Antidiabetic plants and their active constituents”. Phytomed. Vol.2, no.2 pp.137-89, 1995.
[17] Coronado G.D, Thompson B, Tejeda S, Godina R “Attitudes and beliefs among mexican americans about type 2 diabetes”. J Health Care Poor Underserved vol.15, no.4,pp.576–88. 2004
[18] Yeh G.Y, Eisenberg D.M, Kaptchuk T.J, Phillips R.S “Systematic review of herbs and dietary supplements for glycemic control in diabetes”. Diabetes Care, vol.26, no.4,pp.1277–94. 2003.
[19] T .Szkudelski , “ The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat”, Pancreas Physiol Res , vol.50, no.6 , pp. 536- 546, 2001.
[20] S.Lensen, “Mechanism of Alloxan and Streptozocin induced diabetes”, diabetologia, vol. 51,no. 2, pp.216-226, 2008.
[21] H .Herck, Van, V. Baumans, C.J.W. Brandt, A.P.M. Hesp, J.H. Sturkenboom, H.A. Van Lith, G. van Tintelen, and A.C. Beynen “Orbital sinus blood sampling in rats as performed by different animal technicians: The influence of technique and expertise”, Laboratory Animals,vol. 32,no.4, pp.377-386, 1998
[22] P .Trinder, “ Determination of glucose in blood using glucoseoxidase with an alternative oxygen acceptor”, Ann Clin Biochem, vol.6, pp.24-27, 1969.
[23] J. K. Fawcett and J. E. Scott, “ A rapid and precise method for the determination of urea”, J Clin Pathol. Vol.13, no.2, pp.156–159, 1960.
[24] P .Fossati, L. Prencipe, and G. Berti, “Use of 3, 5-Dichloro- 2-hydroxybenzenesulfonic Acid/4-Ami nophenazone Chromogenic System in Direct Enzymic Assay of Uric Acid in Serum and Urine”, Clin. Chem, vol. 26, no.2, pp. 227-231, 1980.
[25] B.T.Doumas, W.A .Watson and H.G.Biggs, “ Albumin standards and the measurement of serum albumin with bromcresol green”, Clin Chem Acta, vol. 31, no.1, pp. 87-96, 1971.
[26] O.H .Lowry, N.J .Rosehmugh , A.L.Farr and K.J .Randall, “Protein measurement with the Folin phenol reagent” , J. Biol Chem. vol.193, pp.265- 275,
[27] R.W. Bonsnes and H.H .Taussky, “On the colorimetric determination of Creatinine by the Jaffe reaction”, J Biol Chem vol.158, pp.581-91, 1945.
[28] H. Ohkawa , N . Ohishi, K. Yagi, “Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction”, Anal Biochem. vol.95, no. 2, pp. 351-358. 1979.
[29] D.E. Kleiner, E.M.Brunt, M.Van Natta, C. Behling, M.J. Contos, O.W. Cummings, L.D. Ferrell, Y.C .Liu, M.S. Torbenson, A. Unalp-Arida, M. .Yeh, A.J. McCullough, A.J .Sanyal, “Non alcoholic steatohepatitis clinical research network, design and validation of a histological scoring system for non alcoholic fatty liver disease”, Hepatology, vol. 41, no.6, pp. 1313–1321, 2005.
[30] E. Friday, Uboh, E. Iniobong Okon, B. Moses, Ekong, “Effect of aqueous extract of psidium guajava Leaves on liver Enzymes, histological integrity and hematological indices in Rats”. Gastroentrology research , vol.3, pp.32-38. 2010
[31] P .Pushparaj, C.H. Tan, B.K Tan , “ Effects of averrhoa bilimbi leaf extract on blood glucose and lipids in streptozotocin-diabetic rats”. J Ethnopharmacol vol.72, pp. 69-76. 2000
[32] K .Rajagopal , K .Sasikala , “Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats”, Singapore Med J. vol.49, no.2, pp. 137-41, 2008.
[33] D.A. Allem, S.Harwood, M. Varagunam, M.J. Raftery, M.M . Yaqoob, “High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases”, FASEB J , vol.17, no.8, pp. 908-910, 2003.
[34] S.S.Chung, E.C. Ho, K.S. Lam, S.K.Chung, “Contribution of poloyl pathway to diabetes-induced oxidative stress”, J Am Soc Nephrol vol.14, no.8, pp.233-236, 2003
[35] M.M. Dallak, P.D. Mikhailidis, A.M. Haidara, M.I. Bin-Jaliah, M.O. Tork, A.M. Rateb, Z.H. Yassin, A.Z. Al-refaie, M.I. Ibrahim, M.S. Elawa, A.L. Rashed, A.N. Afifi, “Oxidative stress as a common mediator for apoptosis induced-cardiac damage in diabetic rats”, Open Cardiovasc Med J. vol.2, pp.70-78, 2008.
[36] Adesokan AA , Akanji MA, A Aderibigbe “Serum glucose and lipid levels in alloxan-induced diabetic rats following oral administration of aloe barbadensis miller juice extract”. Tropical Journal of Health Sciences, vol.13, no.2,pp. 11-14. 2006.
[37] SABU M. Chacko , T. Sabitha , Ramadasan Kuttan, “Amelioration of alloxan-induced hyperglycaemia by aloe arborescens miller. and its possible mechanism”. Pharmacologyonline, vol,2, pp. 112-125. 2008.
[38] EAK, Mohamed , “Antidiabetic, antihypercholesterolemic and antioxidative effect of aloe vera gel extract in alloxan induced diabetic rats”. Aus. J. Basic Appl. Sci vol.5, pp.1321- 1327. 2011.
[39] Duncan Mwangangi Matheka and Faraj Omar Alkizim “Complementary and alternative medicine for type 2 diabetes mellitus: Role of medicinal herbs”. Journal of Diabetes and Endocrinology vol.3, no.4,pp.44-56. 2012
[40] Ayesha Noor S, Gunasekaran A, Manickam S, Vijayalakshmi MA “Antidiabetic activity of aloe vera and histology of organs in streptozotocin induced diabetic rats”. Curr Sci vol.94, no.8,pp.1070-1076. 2008.
[41] Latha M, Pari L. Antihyperglycemic effect of Cassia auriculata in experimental diabetes and its effect on key metabolic enzymes in carbohydrate metabolism. Clin Exp Pharmacol Physiol. vol.30no.1-2,pp.38-43, 2003.
[41]R. B, Kameswara, M.M ,Kesavulu, R .Giri, C.H Appa Rao, “Antidiabetic and hypolipidemic effect of Momordica cymbalaria hook fruit powder in alloxan diabetic rats”. J Ethnopharmacol.;vol.67, no.1,pp.103-109, 1999.
[43]B. K, Chakravarthy, S. Gupta, S.S .Gambir, K.D Gode. “Pancreaticβ-cell regeneration. A novel antidiabetic mechanism of Pterocarpus marsupium Roxb”. Int J Pharm. vol.12, no. 2, pp.123-127. 1980
[44]R.G, M . Macia, P .Ruggenenti “Prevention and treatment of diabetic renal disease in type 2 diabetes: The BENEDICT study”. Journal of the American Society of Nephrology vol.17, no.4-2, pp.S90-97, 2006.
[45] de Zeeuw D “Albuminuria: a target for treatment of type 2 diabetic nephropathy”. Seminars in Nephrology vol.27, no.2, pp.172-181.2007.
[46] T. Zipp, J.R ,Schelling “Diabetic nephropathy. In: Nephrology Secrets (D Hricik, RT Miller, JR Sedor, eds.)” p 105- 108. Hanley Belfus, Philadelphia. 2003.
[47] A. Eidi, M Eidi and M .Sokhteh, “Effect of fenugreek (Trigonella foenum-graecum L.) seeds on serum parameters in normal and streptozotocin-induced diabetic rats”, Nutrition Research, vol.27, no.11, pp.728-733, 2007.
[45]R .S. Kumar and D. Kumar, “ Antidiabetic effect of euphorbia hirta leaves in alloxan induced diabetic mice”, Pharmacologyonline.vol.1 pp.61-69, 2010.
[49] S. Ahmed, M.A. Awal, M.M. Rahman and M .Mostofa ,“Comparative Efficacy of Neem and Karela with insulin and Glibenclamide on Biochemical Parameters in Rabbit,” J. Anima. And Veter. Advan, vol.4,no.2, pp. 221-223, 2005.
[50] P.D. Mayne, “Lipid metabolism”, Clinical Chemistry in Diagnosis and Treatment 6th edition. Clays publisher Ltd. London.; pp 240- 243. 1993.
[51] V.Jaspreet , S. Sivakami, S. Shahani, A.C. Suthar, M.M. Banaralikar and M.K.Biyani, “Antihyperglycemic effect of three extract from Momordica charantia. J. Ethnopharmacol,vol.88,no.1, pp.107-111.2000.
[52] A. Aloulou , K. Hamden, D. Elloumi, M. B. Ali , K. Hargafi , B . Jaouadi, F. Ayadi, A. Elfeki and E. Ammar: “Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats”, BMC Complementary and Alternative Medicine vol.16, no.63, 2012
[53] N. Hfaiedh , J .C. Murat and A. Elfeki, “ Diabetes-Induced Damages in rat kidney and brain and protective effects of natural antioxidants”, J Nutr Food Sci vol.3, no.4, 2013.
[54] M . G. Moghaddam , I. Ansari , M.Roghani , M. Moradi “The Effects of Origanum Majorana on oxidative stress and histopathology of renal tissue among streptozotocin-induced diabetic rat”,. Thrita Journal of Medical Sciences. vol.2, no.1, pp. 29-34,2013.
[55] A.C .Guyton, J.E .Hall, “Textbook of medical physiology”, 10thed. Philadelphia: WB Saundersp, pp.810-818. 2000.
[56] G.L .Bakris, “Diabetic nephropathy. What you need to know to preserve kidney function”, Postgrad Med, vol.93, no.5, pp.89-90, 93-5, 99-100. 1993.
[57] T. Tuvemo, U. Ewald, M. Kobboh, L.A .Proos , “Serum magnesium and protein concentrations during the first five years of insulin-dependent diabetes in children”, Acta Paediatr Suppl, vol. 418, pp.7-10.1997.
[58] N. Makare , S. Bodhankar and V. Rangari , “Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice”, J Ethnopharmacol. vol.78, no.2-3, pp.133–137, 2001
[59] J.P. Almdal and H .Vilstrup , “ Strict insulin therapy normalizes organ nitrogen contents and the capacity of urea nitrogen synthesis in experimental diabetes in rats”, Diabetologia, vol.31, no.2, pp.114-118. 1988.
[60] A. Bener, M. Zirie and R .AI-Rikabi, “Genetics, Obesity, and Environmental Risk Factors Associated with Type 2 Diabetes”, Croat Med J. vol.46, no.2, pp.302-307. 2005.
[61] J.L. Evans, I.D. Goldfine, B.A Maddux , G.M . Grodsky, “Oxidative stress and stress-activated signalling pathways: a unifying hypothesis of type 2 diabetes”, Endocr. Rev vol.23, no.5, pp.599-622, 2002.
[62] R .Rahimi , S .Nikfar, B .Larijani, M .Abdollahi, “A review on the role of antioxidants in the management of diabetes and its complications,” Biomed Pharmacother , vol.59,no.7, pp.365-373. 2005.
[63] Y.Y .Soon and B.K .Tan, “Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats ,” Singapore Med J, vol. 43,no.2, pp. 77-85. 2002.
[64] D .Sathishsekar, S. Subramanian , “Antioxidant properties of momordica Charantia (bitter gourd) seeds on streptozotocin induced diabetic rats”, Asia Pacific J Clin Nut, vol.14 no.2,pp.153-158. 2005.
[65] X.F .Zhang, B.K .Tan,“Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats”, Clin Exp Pharmacol Physiol, vol.27 , no.5-6, pp. 358-363. 2000.
[66] A. Kalaivani1, A. Umamaheswari , A. Vinayagam , and K.Kalaivani , “Anti-hyperglycemic and antioxidant properties of Cassia auriculata leaves and flowers on alloxan induced diabetic rats,” Pharmacologyonline,vol.1, pp. 204-217. 2008.
