Dattatraya Kature*, Gaurav Gupta, Ritu Gilotra
School of Pharmacy, Suresh Gyan Vihar University, Jaipur, Rajasthan-302017, India
ABSTRACT
Traditional medicine (herbs) is an important part of life in developing world. The plant contains various secondary metabolites, some of exhibits proved antimicrobial properties that can beneficial in antimicrobial treatments. The aim of this study is to examine the antimicrobial activity of hydroethanolic leaf extract of Grewia hirsuta at various concentrations against Staphylococcus aureus and Escherichia coli by agar well diffusion method. The plant extract used for qualitative phytochemical investigation by applying standard procedure. Phytochemical screening reveals the presences of alkaloids, glycosides, flavonoids, phenol, proteins saponins and diterpins. Findings of this study indicate that hydroalcoholic extract of Grewia hirsuta have antibacterial activity against the different tested bacterial strains.
Keywords: Grewia hirsuta, Phytochemical Analysis, Hydroalcoholic Extract, Antibacterial Activity.
INTRODUCTION
According to World Health Organization, around 80% of the world’s population prefers medicinal plant extract or their ingredient for primary health care1 because of plant compounds exhibit lesser side effects and potential to replace chemical drugs2. Grewia hirsuta plant is classified under Tiliaceae family and genus Grewia. The plant appears like shrubs and small trees, which distributed in warm climate and many part of the world. In India nearly forty species have been found in India, some of which exhibits medicinal properties3-5. Grewia hirsuta Vahl, commonly known as Nagbala6, traditionally use in headache, rheumatism, joint pain, cholera, sore, diarrhea7. Grewia hirsuta plant’s compounds are mainly oleic acid, linoleic acid, linolelaidic acid, terpenes, saturated fatty such as myristic acid, palmitic acid, undecanoic acid, gingerol, aldehyde and alcoholic compound8.
Although environmental factors affects on production of active ingredients, controlled by genetic process in medicinal and cause changes in growth, quantity and quality of their active ingredients, such as alkaloids, glycosides, steroids, and essential oils of medicinal plants9. In natural ecosystems, environmental factors such as climate, soil, geographic location can have a major impact on increasing or decreasing the quantity and quality of plan ingredient10. Hence, the present investigation was aimed for phytochemical analysis and in-vitro study of antibacterial activity of the hydroethanolic leaf extract of Grewia hirsuta plant collected from Western Ghats forest.
MATERIALS AND METHODS:
Plant Material
Grewia hirsuta’s plant leaves collected from Western Ghats forest of Belgavi, Karnataka, India. Plant identification and authentification was taxonomist from “ICMR- National Institute of Traditional Medicine, Belagavi, Karnataka, India”.
Preparation of plant extract
The polar organic solvent extraction process was followed for preparation of extract11. The leaves were shed dried and powdered. Approximately 150 gm of powdered leaves were exhaustively extracted with hydroalcoholic solvent (70:30: Methanol: Water) by maceration method and extract was evaporated above their boiling points 12.
Qualitative Phytochemical Analysis
The plant extract was screened to identify the existence of primary and secondary metabolites, like alkaloids, glycosides, flavonoids, tannins, saponins, terpenoids, proteins and fixed oils, by standard screening test and phytochemical procedures 13, 14.
Thin layer chromatography
The hydroethanolic solvent extract was used for thin layer chromatography (TLC) study. The method adopted was ‘conventional one dimensional ascending technique’ using silica gel 60F254, and the size 7X6 cm, from Merck. The markings were made and using glass capillaries sample was spotted on plate. The sample volumes used 1µl at distance of 1 cm and plate immerse in to the developer. A mixture of Toluene: Ethyl acetate (9:1), Chloroform: methanol (9:1), Ethyl acetate: Methanol (5:5), Toluene: Ethyl acetate (6:4), Toluene: Ethyl acetate (5:5), Toluene: Ethyl acetate (4:6) used as developer. The dried plates were sprayed freshly prepared iodine reagents to spot the bands on the TLC plates. The flow of compound through column was measured by its retention factor (Rf). Once the chromatogram was developed the Rf Value of the spot was calculated using the formula [15].
Rf = Distance travelled by solute / Distance travelled by solvent”
Test Microorganisms:
The test organism used in this study namely Staphylococcus aureus & Escherichia coli were obtained from, “National Centre for Cell Science, Pune, Maharashtra, India”. Media used for the antibacterial test was Nutrient Agar/Broth and procured from Hi-Media Pvt. Ltd, Mumbai, India.
Method of preparation
The agar medium was dissolved in distilled water and boiled in conical flask of sufficient capacity. The dry ingredients were dissolved in adequate quantity of distilled water dissolve the medium completely by applying heat. The Sterilization culture media were performed as flask containing medium was cotton plugged and placed in autoclave for sterilization at 15 lbs /inch2 at 1210C for 15 minutes. The media in flask was immediately poured (20 ml/ plate) into sterile petri dishes on plane surface prior to sterilization. Then, plates were left at room temperature to solidify and incubate at 370C overnight to check the sterility of plates.
Antibiogram Test
The agar “well diffusion method” was used to determine the “antimicrobial activity of plant extract”16. On solidify agar medium, wells of 4 mm diameter were prepared by using sterile cork borer under sterile conditions. These wells were filled with plant extract of the various concentrations of 25, 50 and 100 mg/ml and the standard drug, ciprofloxacine antibiotics of 10, 20 & 30 μg/ml concentration were used as positive controls, and then plates were kept for incubation at 37˚C for 24 hrs. The zone of inhibition (in mm) technique were used for assess the antibacterial activity against test organisms.
Statistical Analysis
The experimental data were expressed as mean ± standard deviation (SD) and data obtained were analyzed using one-way analysis of variance (ANOVA).
RESULT
Estimation of Phytochemical Constituents
The Grewia hirsuta exhibited the presence of important biologically active secondary metabolites, like saponins, phenols, alkaloids, ditrepins, flavonoids, glycosides, carbohydrates, and proteins which were established that demonstrated in Table 1.
Table 1: Phytochemical analysis of Grewia hirsuta leaf extracts.
| Phytochemical Constituents | Grewia hirsuta Hydroethanolic Plant Extract |
| Test for Alkaloids
Hager’s test |
++ |
| Glycosides
Legal’s test |
++ |
| Flavonoids
Lead acetate |
+++ |
| Phenols
Ferric Chloride Test |
++ |
| Proteins
Xanthoproteic test |
+ |
| Carbohydrates
Fehling’s test |
+ |
| Saponins
Froth Test |
+ |
where, +: present (mild “amount), ++: present (moderate amount), +++: present”
(large “amount), −: absent, based on the” power of generated “color reaction”.
Thin layer chromatography (TLC)
The TLC results are shown in Fig. 1. The toluene: ethyl acetate (6:4) sample is optimized mobile phase is used to isolate flavonoid from hydroalcoholic extract. Finding shows that the hydroalcoholic extract, fraction of toluene: ethyl acetate (6:4) fraction had three spots while the other fraction had one spot. In hydroalcoholic extract toluene: ethyl acetate (6:4) fraction the visible yellow color of the compound spotted with purple background exhibited compounds that have antibacterial activity against selected microorganism. Profile of spot and Rf value of chromatography results is shown in Table 2.
Table 2: Rf value of Toluene: Ethyl acetate (6:4) optimized mobile phase of plant extract.
| Mobile phase | Rf value |
| Toluene: Ethyl acetate (6:4) optimized mobile phase
Dis. travel by mobile phase=5.5cm No. of spot at long nm=5 No. of spot at short nm=3 No. of spot at normal light=2 |
0.10,0.2,0.38,0.47,0.90 0.2,0.30,0.90 0.30,0.90 |
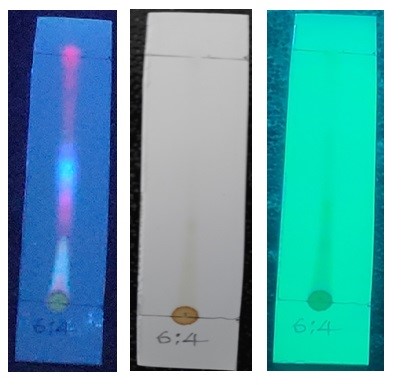
Fig. 1: Thin layer chromatography of Grewia hirsuta sample in toluene:ethyl acetate (4:4) mobile phase.
Antibiogram Test:
The antibacterial profile of the standard drug Ciprofloxacin and hydroethanolic extract of Grewia hirsuta leaves and against Staphylococcus aureus and E. Coli microorganism. The zone of inhibition of standard drug at various doses against microorganism shown in table 3. The plant extract at various doses tested on microorganism and the dose at 100 mg/ml revealed satisfactory activity against Staphylococcus aureus and E. Coli with zone of inhibition 19±0.47 mm and 18±0.28 mm. In comparison to the standard drug, antibacterial activity of plant extract at dose of 100 mg/ml was established. The Photoplates for standard drug and hydroethanolic plant extract presented in figure 2 and figure 3.
Table 3: “Antimicrobial activity” of “standard drug” against selected microbes”
| Sr. No. | Name of drug | Microbes | “Zone of inhibition” | ||
| 10 μg/ml | 20 μg/ml | 30 μg/ml | |||
| 1. | Ciprofloxacin
|
Staphylococcus aureus” | 17±1.69 | 18±2.62 | 22±2.16 |
| E. Coli” | 12±0.28 | 15±0.57 | 18±0.28 | ||
*(n=3, mean ± SD)
Table 4: “Antimicrobial activity” of hydroalcoholic extract against” selected microbes
| Sr. No. | Name of microbes | “Zone of inhibition” | ||
| Hydroethanolic extract | ||||
| 25mg/ml | 50 mg/ml | 100mg/ml” | ||
| 1. | Staphylococcus aureus” | 8±0.47 | 11±0.47 | 19±0.47 |
| 2. | E. Coli” | 6±0 | 7±0.57 | 16±2.05 |
*(n=3, mean ± SD)
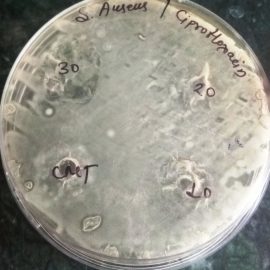
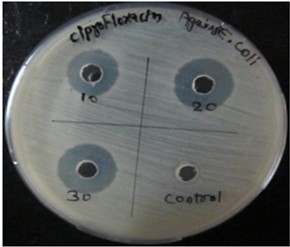
Fig 2: Photoplates of Antimicrobial activity of standard drug against selected microbes

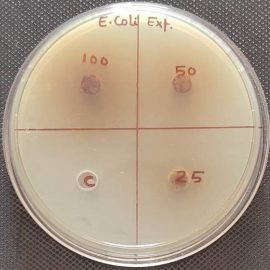
Fig 3: Photoplates of antimicrobial activity of hydroalcoholic extract against selected microbes
DISCUSSION
World is experiencing increasing number of multidrug-resistant microorganisms and their detrimental effect on health of human being. Various studies are going on to find out new compounds from plant origin that are of useful for treatment for various diseases 17. The plants exhibit presence of various bioactive compounds with extensive medical importance18 such as phenolics and flavonoids compounds which possesses antioxidant and antimicrobial properties19. The plant was collected from the Western Ghats forest of Belgum, Karnataka province. The plant collected from specific location because, effect of various environmental factors like soil, temperature, climate etc on growth and the phytoconstituents quality and quantity present in the plant10. Plants naturally grown in forest containing more potent and high quantity of phytoconstituents as compare to plant grown in garden. So, this study has evaluated the antimicrobial activity of hydroethanolic leaf extract of Grewia hirsuta a collected from Western Ghats forest.
CONCLUSION
This research demonstrates the antimicrobial potential of leaves from Grewia hirsuta. The hydroethanolic leaf extract were active against Staphylococcus aureus, gram-positive bacteria and E-coli, gram-negative bacteria and inhibit their growth. It is need to find out the active compound present in plant extract by doing further study, isolation and identification of the active compounds which possibly will be used as lead molecule in the development of new antibacterial drugs.
ACKNOWLEDGEMENT
The authors are thankful to the Principal and Management of Delonix Society’s Baramati College of Pharmacy, Baramati, Pune, Maharashtra for providing facilities and support.
REFERENCES:
- Noguchi N, Niki E. Phenolic antioxidants: A rationale for design and evaluation of novel antioxidant drug for atherosclerosis. Free Rad Biol. Med. 2000; 28: 1538–1546.
- Berahou A. Antibacterial activity of Quercus ilex bark’s extracts. Jr. of Ethno. 2007; 112: 426–429.
- Kirtikar KR, Basu BD. Indian Medicinal Plants. Basu LM and Co. Allahabad. 1975.
- The Wealth of India, Raw Materials and Industrial Products. CSIR. New Delhi. 1956; 4:266.
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. CSIR. New Delhi. 1956.
- Sharma AK. Medicinal properties of bala (Sidacordi folialinn and its species). Int Jr Ayurveda Pharma Res. 2013; 02:1–9.
- Goyal PK. Phytochemical and pharmacological properties of the genus Grewia: a review. Int J Pharm Pharm Sci. 2012; 4:72–78.
- Natarajan A, Sugumar S, Bitragunta S, Balasubramanyan N. Molecular docking studies of (4 Z, 12 Z)-cyclopentadeca-4, 12-dienone from Grewiahirsuta with some targets related to type 2 diabetes. BMC Complement Altern Med. 2015; 73:1–8.
- Omidbeigy R. Approaches to the production and processing of medicinal plants. Vol. II, 42, Designers Publishing. 1995.
- Lebaschi MJ, Sharifi AA. Changes in Hypericin in different habitats of Goli. Research of Iranian Medicinal Plants and Herbs. Res Inst. Fore. Rang. 2001; 11: 100–87.
- Sarkar SD, Zahid L, Grey AI. Methods in Biotechnology. Natural Product Isolation. Second edition; Humana Press. 2005.
- Mukherjee PK. Quality Control of Herbal Drugs. 2nd Edition, Business Horizons. 2007.
- Roopashree TS, Dang R, Shobha RH, Narendra C. Antibacterial activity of anti-psoriatic herbs: Cassia tora, Momordica charantia and Calendula officinalis. Int Jr of App Res in Natural Product. 2008; 1(3): 20-28.
- Obasi NL, Anthony CC, Ogwuche P. Comparative phytochemical and antimicrobial screening of some solvent extracts of Samanea saman pods. Afri jr of pure and applied chem. 2010; 4(9): 206-212.
- Bhattarai HD, Babita P, Hong SG, Hong KL, Joung HY. Thin layer chromatography analysis of antioxidant constituents of lichens from Antarctica. J Nat Med. 2008; 62:481–484.
- Perez C, Pauli M, Bazerque P. An Antibiotic Assay by Agar Well Diffusion Method. Acta Biologiae et Medicinae Experimentalis. 1990; 15: 113-115.
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization. 2014.
- Harvey AL, Edrada-Ebel R, Quinn RJ. There emergence of natural products for drug discovery in the genomics era. Rev.Drug Discov. 2015; 14:111–129.
- Arulmozhi S, Mazumder PM, Narayanan LS, Thakurdesai PA. In vitro antioxidant and free radical scavenging activity of fractions from Alstonia scholaris Linn.R.Br. Int J Pharm Tech Res. 2010; 2(1):18-25.
