Mr. Sujit Desai*2, Dr. Preeti Khulbe1, Dr. Arehalli Manjappa3
- Gyan Vihar School of Pharmacy, Suresh Gyan Vihar University, Jaipur, Rajasthan, India,
- Annasaheb Dange College of D Pharmacy, Ashta, Maharashtra, India.
- Tatyasaheb Kore College of Pharmacy, Warananagar, Maharashtra, India
ABSTRACT
Malignancy speaks to a gathering of heterogeneous ailments described by uncontrolled development and spread of abnormal cells, at last leading to death. Nanomedicine assumes a huge job in the advancement of nanodrugs, nanodevices, drug delivery systems as well as nanocarriers. A portion of the significant issues in the treatment of cancer are multidrug resistance (MDR), restricted helpful window and undesired symptoms of accessible anticancer drug and the constraints of anticancer drugs. A few nano systems being used for recognition, determination and treatment, for example, theranostic bearers, liposomes, carbon nanotubes, quantum spots, polymeric micelles, dendrimers and metallic nanoparticles. Nonetheless, non-biodegradable nanoparticles cause high tissue aggregation and prompts harmfulness. MDR is viewed as a significant obstruction to disease treatment because of metastatic tumors that create protection from chemotherapy. MDR adds to the disappointment of chemotherapies in different diseases, including bosom, ovarian, lung, gastrointestinal and hematological malignancies. In addition, the helpful proficiency of anticancer medications or nanoparticles (NPs) utilized alone is not as much as that of the blend of NPs and anticancer drugs. Blend treatment has for quite some time been received first choice of the treatment of a few malignancies to improve the clinical result. Combination treatment with anticancer medications has been appeared to for the most part instigate synergistic medication activities and discourage the beginning of medication opposition. We initially give a far reaching outline of the angiogenesis and of the various kinds of NPs at present utilized in medicines of malignant growth; those accentuated in this survey are liposomal system, polymeric nanoparticles, polymeric micelles PMs, dendrimers, carbon nanoparticles, nanodiamond ND, fullerenes, carbon nanotubes CNTs, graphene oxide GO, GO nanostructured and metallic nanoparticles utilized for combination treatment with different anticancer agent. Nanotechnology has given the advantageous tools to combination treatment. However, for clinical interpretation, we need proceeded with enhancements in the field of nanotechnology.
Keywords: liposomes; polymeric nanoparticles; dendrimers; carbon nanoparticles; graphene oxide nanocomposites; metallic nanoparticles; anticancer drugs; Combination treatment.
INTRODUCTION
Chemotherapy is a significant methodology in malignant growth treatment. Compelling treatment of malignant growths needs precise delivery of an enough intracellular portion of chemo-medications to slaughter the disease cells [1]. What’s more, chemotherapy for malignancy is a fragile harmony among reaction and harmfulness, while low-dosing neglects to acquire compelling impacts, over-dosing prompts exorbitant foundational poisonousness [2]. Besides, tranquilize appropriation proficiency from plasma to tumors is influenced by some physiologic parameters, for example, serious medication take-up by liver, discharge of little atom sedates by pee, medicate inactivation by official to proteins, and low stability of medication in liquids [3]. Along these lines, Nanoscale drug delivery systems have been widely studied in recent years for tumor-targeted drug treatment because of their possibilities to upgrade and safeguard the clinical helpful impacts of chemo-drugs with fewer symptoms by improving their protection, absorption, penetration and distribution [2, 4–6]. Nano carriers for medicate delivery have a few focal points [2, 7, 8] (1) shielding the medication from being debased and dragging out the maintenance time in the body; (2) expanding the solvency of some hydrophobic medications; (3) directed conveyance and controlled arrival of medications by nanoparticles alteration to keep the medication focus in tumor destinations and boost restorative impacts; (4) possibility of different medication delivery to accomplish synergistic remedial reaction, or use of combination treatment, for example, chemo-photothermal treatment. Osteosarcoma is the most widely recognized essential harmful bone tumor usually happened in youth and immaturity [9]. Because of the presentation of chemotherapy and advances in careful innovation, the 5-year endurance pace of those with nearby osteosarcoma has improved to estimated 70% [9, 10]. Notwithstanding, current chemo-sedates regularly utilized in treatment of osteosarcoma are restricted for their symptoms and improvement of opposition [11]. To deliver these disadvantages and to expand the viability of chemotherapy, an assortment of nanoplatforms for focused medication or quality conveyance has been broadly examined in osteosarcoma, and nanotechnology has been proposed as a promising methodology for osteosarcoma treatment [7, 12–14]. In this survey, we reflectively summed up the ongoing advances of nanocarriers for focused medication conveyance in osteosarcoma. We talk about the usually utilized kinds of medication conveyance nanoparticles, controlled medication discharge upon various upgrades, and nanocarriers alteration procedures for focused medication conveyance. The utilization of nanoparticles in the administration of metastatic osteosarcoma is additionally quickly examined. Also, we trust this audit will give perusers a general comprehension of current status in osteosarcoma nanomedicine, and motivate further examinations in novel medication conveyance nanosystems for osteosarcoma treatment.
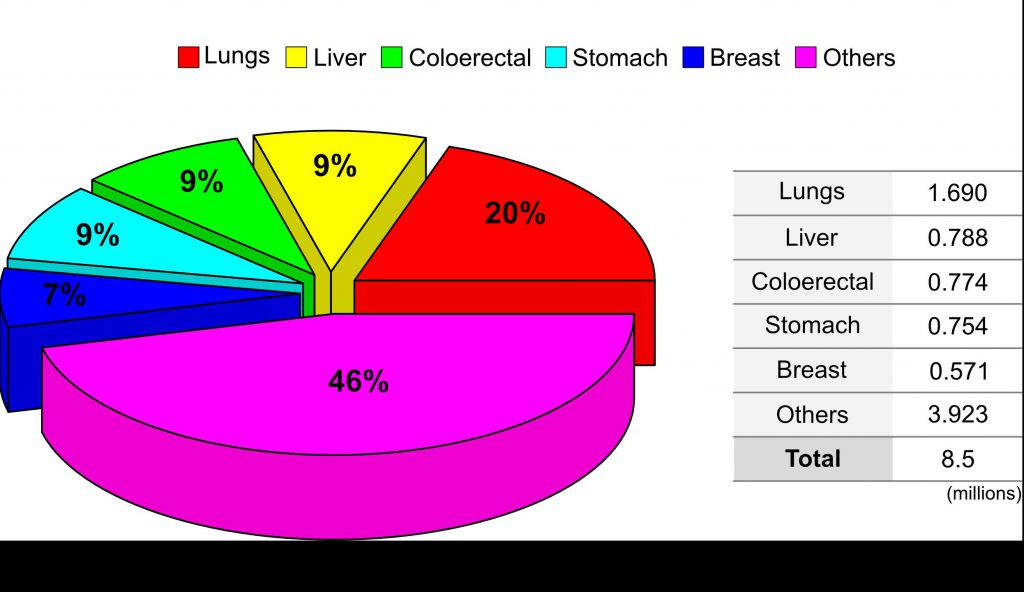
Fig 1 : The world wide percentage distribution of cancer type.
Cancer deaths have increased at an alarming rate in both developed as well as developing countries.(https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts figures/cancerfacts-). Currently, cancers are being treated with a variety of treatments including chemotherapy, hormone therapy, radiation, surgery, immune therapy and targeted therapy [3,4]. Thusly, further mediations are important to bring down the rate and deal with the malady by executing different techniques, including new treatment strategies. Albeit different helpful modalities are accessible, it is basic to distinguish the best treatment. Despite the fact that the regular treatments have improved patients’ endurance, they additionally have a few restrictions [5], for example, vague focusing on, sedate opposition, extreme poisonous impacts and undesired reactions. Among a few new helpful methodologies, nanotechnology in malignancy assumes a few jobs including the recognition and treatment of disease, distinguishing proof of biomarkers, comprehension of disease progress and managing the improvement of new diagnostics and imaging specialists [6]. Angiogenesis is an essential, mind boggling, controlled and coordinated arrangement of occasions including development of fresh blood vessels from the previous ones, which is a sign of tumor advancement and metastasis [7,8]. Angiogenesis is an inescapable procedure for tumor development, attack and metastatic dispersal [7]. Physiological angiogenesis of multiplying endothelial cells in the ovaries, uterus and placenta is restricted, though obsessive angiogenesis is incredible in malignant growth and in the wound healing procedure [9], where it is fundamentally constrained by the degree of genius angiogenic and hostile to angiogenic variables and coordination among various sorts of cells including macrophages, endothelial cells and pericytes [7,8,10]. Angiogenesis is a basic procedure in malignant growth and in diabetic retinopathy, in which the cells are portrayed by unusual vasculature structure and capacities, for example, convoluted, widened, saccular vessels and high penetrability [7,8,10,11]. The irregular vascular structure prompts expanded degrees of vascular endothelial development factor (VEGF) and diminishes the adequacy of cytotoxic chemotherapy [8,12]. Among a few development factors, VEGF assumes a key job in guideline, both in ordinary and malignant growth cells, advancing endothelial cell relocation, expansion and slim cylinder arrangement. The raised degree of VEGF is viewed as a main consideration for tumor arrangement and unusual vessel development [12]. Controlling tumor angiogenesis is a fundamental piece of the host barrier framework and is managed by angiogenesis inhibitors, for example, endostatin and thrombospondin-1; the awkwardness among inhibitors and development factors prompts angiogenesis [13–15]. Various modalities have been created for angiogenesis-related illnesses to forestall new vessel development in tumors and diabetic retinopathy, including: preclinical and clinical preliminaries have tried VEGF antibodies as a treatment [16]; an angiogenesis inhibitor can execute profoundly proliferative tumor cells by denying the tumors of and oxygen [17]; bevacizumab, an enemy of VEGF counter acting agent, fundamentally builds by and large endurance of patients with customary chemotherapeutic specialists for various kind of malignancies [18]; and Sunitinib, an enemy of angiogenic sedate that restrains the VEGF receptor (VEGFR) tyrosine kinase and Sorafenib, an enemy of angiogenic RTKI, show unrivaled action in patients with renal cell carcinoma when contrasted with the standard of care interferon-treatment.[19,20]
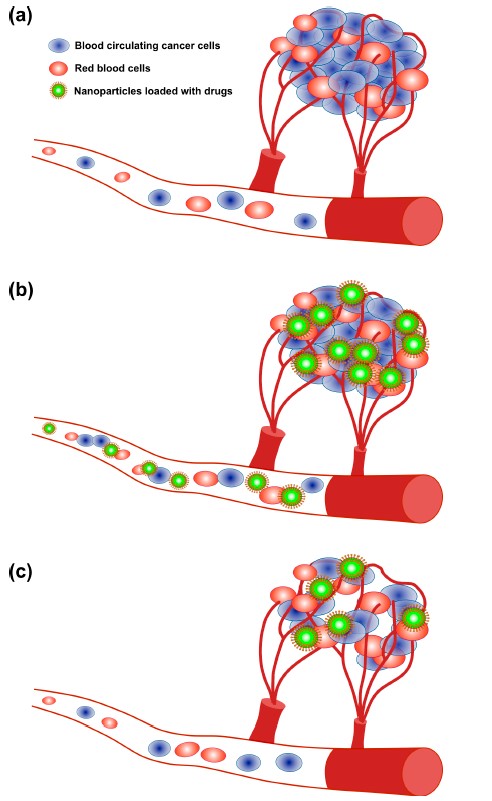
Fig 2: The angiogenesis process and treatment of tumors via nanoparticle delivery. (a) Angiogenesis; (b) Drug delivery through NPs; (c) Tumor reduction
Albeit different advancements have been created to help in understanding disease science and anticancer therapeutics, the death rates for some, regular malignant growths have improved just marginally in the previous two decades. In this way, imaginative methodologies are expected to improve the viability and the remedial record of anticancer treatment [21]. Focusing on medicines against strong tumors by an assortment of modalities prompts sensational relapses; notwithstanding, the impact is just present moment because of advancement of protection from the treatment. In this manner, the utilization of different restorative anticancer agents in blend with NPs is promising methodology to treat tranquilize safe disease. Nanomaterials-based malignant growth treatment assumes a significant job in expanding the restorative proficiency against disease by using a blend of nanomaterials and chemotherapeutic agents;
Combination treatment has been appeared to build tumor control and decrease undesired symptoms by improving the pharmacokinetics and focused delivery of the medication payloads [21,22]. Combination treatment is standard practice in regular chemotherapy to defeat cross-opposition and accomplish synergistically improved helpful result without essentially expanding poison levels. By and large, monotherapy depends on utilization of a solitary medication, which isn’t adequate to achieve tumor relapse. With blend chemotherapy, the synergistic impact of (at least two) operators focusing on various malady pathways, qualities, or cell-cycle checkpoints in the disease procedure are utilized to raise the odds of dispensing with malignant growth [23]. Accordingly, blend treatment is an appropriate and elective method of treatment and a promising way to deal with improve the adequacy of malignancy treatment. NP-intervened combination treatment would convey numerous restorative agent with various physico-compound and pharmacological properties in single NPs. NPs can keep up the streamlined synergistic medication proportion in a solitary bearer right to the point of intracellular take-up by the objective malignant growth cell. By and by, combination chemotherapy (“two-in-one” approach) brings about a superior reaction and an improved endurance contrasted and single-specialist treatment for clinical and preclinical examinations [23]. During the most recent two decades, nanotechnology has gotten huge consideration and has added to clinical therapeutics. Especially, the advances in biocompatible nanoscale medicate bearers, for example, liposomes and polymeric NPs, have empowered more secure and increasingly effective delivery of a bunch of medications. NPs are promising medication transporters due to their capacity to convey hydrophobic or potentially hydrophilic medication atoms, peptides, little particle medications, or antibodies to the tumor site with least harmfulness to encompassing tissues; this protected conveyance depends on their entrance limit [24]. The potential anticancer properties of chemotherapeutic operators’ capacity and conveyance have been investigated utilizing different sorts of NPs, including especially liposomes, polymeric NPs, PMs, dendrimers, carbon NPs, polymer-sedate conjugates, nanoemulsions, CNTs, quantum specks and inorganic NPs (Figure 3). As of late, theranostic nanocarriers have indicated a lot of enthusiasm for different treatment because of their capacity to execute synchronous capacities at focused sick destinations. Nanotheranostic bearers made out of metallic or attractive nanoparticles. What’s more, we clarified combinatorial parts of nanoparticles as well as the method of activity individual cytotoxic operator. Thus, we clarified combinatorial parts of nanoparticles as well as the method of activity individual cytotoxic agent.
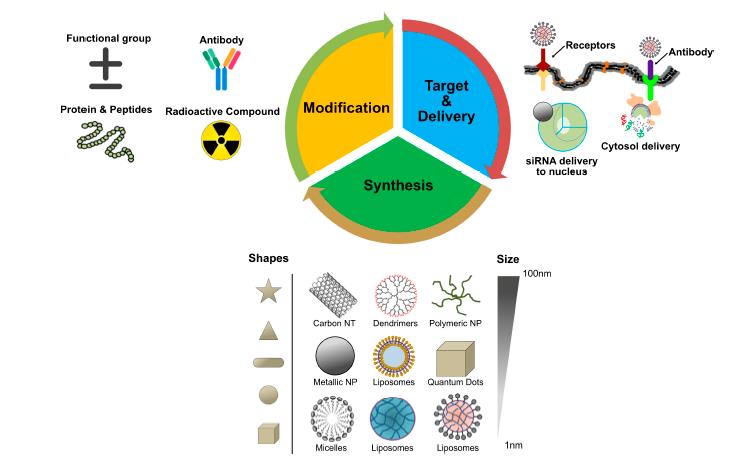
Fig 3: Central dogma of nanoparticles targeting in cancer cells.
Types of NPs Used for Combination Therapy
- Liposomes
The development of an nanotechnology had a noteworthy effect on clinical therapeutics over the most recent two decades. Liposomes and polymeric nanoparticles have been utilized as nanoscale medicate bearers for progressively effective and more secure delivery of incalculable medications. Among various assortments of NPs, the liposomes are the most settled medication conveyance vehicle, with numerous clinical items accessible to date. Liposomes comprise of amphiphilic lipid atoms that gather into bi-layered round vesicles [26,27]. Liposome-interceded medicate conveyance is an improvement because of their effectiveness, bio-similarity, non-immunogenicity, upgraded solvency of chemotherapeutic specialists and their capacity to embody a wide exhibit of medications [28]. Moreover, liposomes have indicated huge restorative potential as bearers for payloads and for conveyance to focused locales [29]; liposome-intervened sedate conveyance frameworks improve the pharmacokinetic and pharmacodynamic profiles of the helpful payload, advance controlled and supported arrival of medications and show lower foundational harmfulness contrasted and the free medication[29].For example, polyethylene glycol (PEG)- covered liposome-interceded sedate conveyance expanded the in vivo course half-existence of liposomes from a couple of hours (h) to roughly 45 h [30]. At present, unique liposomal items being utilized for malignant growth treatment incorporate Doxil, DaunoXome®, DepoCyt® and ONCO-TCS, which are liposomal definitions of doxorubicin (DOX), daunorubicin, cytarabine and vincristine, separately [30]. Lammers et al. (2009) exhibited that all the while conveying different chemotherapeutic specialists to tumors in vivo utilizing HPMA-based polymer-medicate conjugates conveying 6.4 weight % of gemcitabine, 5.7 weight % of DOX and 1.0 molar % of tyrosinamide. The subsequent develop, poly(HPMA-co-MA-GFLG-gemcitabineco-MA-GFLG-DOX-co-MA-TyrNH2), was demonstrated to be powerful at killing malignant growth cells under in vitro conditions; moreover, flowing time was drawn out and confinement proficiency to restrain tumor development was exceptionally specific [30]. With respect to interceded treatment, different procedures have been received for uninvolved focusing of liposomes to the tumor destinations by utilizing PEG and dynamic focusing of malignant growth cell surface receptors, FRs, TfR and EGFRs and the tumor microenvironment including VEGF, VCAM, grid.
Lipids were the first self-assembled materials used for drug delivery, mainly in the form of micelles and liposomes [30]. Liposomes are arranged based on size and the quantity of phospholipid film layers [31,32], for example, multilamellar vesicles (MLV) with a normal size somewhere in the range of 1 and 5 m, huge unilamellar vesicles (LUV) in the size scope of 100–250 nm with a solitary lipid bilayer and little unilamellar vesicles (SUV) comprising of a solitary phospholipid bilayer encompassed by a watery stage with a size scope of 20–100 nm [30].
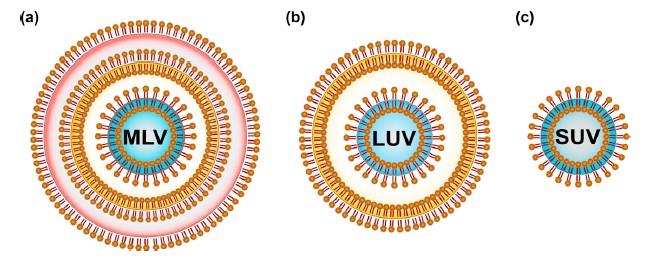
Figure 4 : Classification of liposomes based on the lamellarity: (a) Multilamellar vesicles (MLV) are composed of many lipid bilayers and range from 1–5 m in size; (b) Large unilamellar vesicles (LUV) are in the size range of 100–250 nm with a single lipid bilayer; (c) Small unilamellar vesicles (SUV) consist of a single phospholipid bilayer surrounding an aqueous phase with a size range of 20–100 nm.
Strategies for osteosarcoma-targeted drug delivery
Targeted drug delivery generally means delivery of the intravenously administered drugs to the target site, e.g. tumors. Targeted drug delivery systems are designed to encourage drug delivery to the tumor locales with least symptoms, and that are performed by two focusing on techniques, including uninvolved and dynamic focusing on [33] (Figure 1). Contrasted and free little restorative operators, nanocarriers can latently amass in tumors through the improved porousness and maintenance (EPR) impact, which is described by flawed veins and debilitated lymphatic waste in tumor tissues, and accomplish unrivaled helpful adequacy, while lessening symptoms [34,35].
Despite the fact that nanocarriers can be latently focused to tumor by means of EPR impact, it experiences some genuine restrictions, for example, wasteful medication dissemination into tumor cells, the arbitrary idea of focusing on, and the absence of EPR impact in certain tumors. In this manner, there is potential to improve the tumor targetability of the nanocarriers through dynamic focusing on systems, for example, ligand-interceded tumor focusing on [34,35].Ligand-functionalized nanocarriers could connect with disease cells and be disguised by means of receptor-intervened endocytosis system, along these lines bringing about higher restorative impact [34,35]. The information on tumor cell epitopes and advances in nanotechnology have permitted the improvement of focused nanocarriers ready to effectively convey antitumor specialists to sick zone. Bisphosphonates [34,35]., aptamers [14], hyaluronic corrosive (HA) [24], folate [34], and peptides [33] have been accounted for to be utilized in structuring osteosarcoma- targeted drug delivery system.
Research Gap
- Lack of availability of right combination of chemotherapy drugs against Bone Cancer (Osteosarcoma).
- Lack of right approaches for effective targeting of drugs to bone.
- No effective nanoparticle approaches to delivery combination of hydrophilic and lipophilic medications to obtain uniform kinetic, dynamic profiles for both the drugs for effective treatment of osteosarcoma.
- Thus, in the current investigation to overcome above challenges, we are proposing to develop Liposomal delivery system for delivery of combination of hydrophilic and liopophilic drugs to osteosarcoma using active targeting approach (surface coated with bone specific compounds such as alendronate sodium).
The foreseen outcomes
- Enhancement in loading of drugs with required physical and chemical properties.
- Adequate in vivo stability (decreased opsonisation and enhanced circulation half-life).
- Decreased premature cargo release.
- Increased tumor distribution of payloads.
- Increased uptake by target cells/tissue.
- Sustained therapeutic effect due to long circulation half-life.
- Increased in-vivo therapeutic efficacy.
CONCLUSIONS
Right now accessible traditional malignancy remedial methodologies experience the ill effects of extreme restrictions for example, bio-distribution, insufficient targeting by the therapeutic agents, poor solubility, poor oral bio-availability, low therapeutic records, portion constraining harmfulness to sound tissues and above all, constantly, almost invariably an emerging drug resistance. Ongoing clinical and preclinical information demonstrated that combination treatment displayed potential applications in malignant growth. NPs are multitalented stages used to act with anticancer medications to improve the remedial exhibitions in malignancy cells through maintaining drug solidness and giving bio-accessibility, long haul maintenance, special gathering of medications in tumor destinations by means of upgraded pervasion and maintenance impacts, decreased symptoms and an expanded helpful record. In the course of the most recent decade, nanoparticle frameworks have been effectively saddled direct anticancer operators or for co-conveyance of various anticancer specialists. The utilization of NPs in mix with other anticancer specialists controls different pathways associated with the different phases of disease cell, including development, movement, metastasis and medication opposition. The exceptional properties of NPs incorporate size, huge surface-to-volume proportion, the capacity to typify an assortment of medications and tunable surface science. Lipid bi-layered vesicles called liposomes are much of the time utilized because of their effectiveness, bio-similarity, non-immunogenicity and their capacities to improve solvency of chemotherapeutic specialists and embody a wide cluster of medications.
In outline, combination treatment gives an alluring methodology to treatment of an assortment of tumors to help beat drug resistance, low adequacy and high-portion initiated fundamental harmfulness. Notwithstanding, handling the difficulties will require gigantic exertion. NP-interceded blend treatment holds incredible guarantee for effective clinical interpretation of nano medicine medications, which will give special advantages to disease patients. Future investigations are justified to address security, biocompatibility, simple accessibility and especially study should concentrate on poisonousness. The most significant part of future examinations is the necessity to address an assortment of creature models and conceivable clinical investigations on people should be performed to validate their utilization in malignant growth treatment.
REFERENCES
- Jemal A, Bray F ,Center M.M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90.
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691.
- American Cancer Society. Cancer Facts & Figures 2016. Available online: https://www.cancer.org/research/ cancer-facts-statistics/all-cancer-facts-figures/cancer facts-figures-2016.html (accessed on 19 July 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
- Nguyen, P.L.; Gu, X.; Lipsitz, S.R.; Choueiri, T.K.; Choi, W.W.; Lei, Y.; Hoffman, K.E.; Hu, J.C. Cost Implications of the Rapid Adoption of Newer Technologies for Treating Prostate Cancer. J. Clin. Oncol. 2011, 29, 1517–1524.
- Mignani, S.; Bryszewska, M.; Klajnert-Maculewicz, B.; Zablocka, M.; Majoral, J.-P. Advances in Combination Therapies Based on Nanoparticles for Efficacious Cancer Treatment: An Analytical Report. Biomacromolecules 2015, 16, 1–27.
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286.
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257.
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111.
- Blau, H.M.; Banfi, A. The well-tempered vessel. Nat. Med. 2001, 7, 532–534.
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989.
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10.
- Sun, J.; Wang, D.; Jain, R.K.; Carie, A.; Paquette, S.; Ennis, E.; Blaskovich, M.A.; Baldini, L.; Coppola, D.; Hamilton, A.D.; et al. Inhibiting angiogenesis and tumorigenesis by a synthetic molecule that blocks binding of both VEGF and PDGF to their receptors. Oncogene 2005, 24, 4701–4709.
- Ma, J.;Waxman, D.J. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol. Cancer Ther. 2008, 7, 3670–3684.
- Sund, M.; Hamano, Y.; Sugimoto, H.; Sudhakar, A.; Soubasakos, M.; Yerramalla, U.; Benjamin, L.E.; Lawler, J.; Kieran, M.; Shah, A.; et al. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc. Natl. Acad. Sci. USA 2005, 102, 2934–2939.
- Heath, V.L.; Bicknell, R. Anticancer strategies involving the vasculature. Nat. Rev. Clin. Oncol. 2009, 6, 395–404.
- Teicher, B.A. A systems approach to cancer therapy. (Antioncogenics + standard cytotoxics–>mechanism(s) of interaction). Cancer Metastasis Rev. 1996, 15, 247–272.
- Rini, B.I.; Tamaskar, I.; Shaheen, P.; Salas, R.; Garcia, J.; Wood, L.; Reddy, S.; Dreicer, R.; Bukowski, R.M. Hypothyroidism in Patients with Metastatic Renal Cell Carcinoma Treated with Sunitinib. JNCI J. Natl. Cancer Inst. 2007, 99, 81–83.
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 115–124.
- Escudier, B.; Eisen, T.; Stadler,W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134.
- He, Y.; Lin, J.; Kong, D.; Huang, M.; Xu, C.; Kim, T.-K.; Etheridge, A.; Luo, Y.; Ding, Y.;Wang, K. Current State of Circulating MicroRNAs as Cancer Biomarkers. Clin. Chem. 2015, 61, 1138–1155.
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782.
- Xu, J.L.; Jin, B.; Ren, Z.H.; Lou, Y.Q.; Zhou, Z.R.; Yang, Q.Z.; Han, B.H. Chemotherapy plus Erlotinib versus Chemotherapy Alone for Treating Advanced Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0131278.
- Jadia, R.; Scandore, C.; Rai, P. Nanoparticles for Effective Combination Therapy of Cancer. Int. J. Nanotechnol. Nanomed. 2017, 1, 1–15.
- Parhi, P.; Mohanty, C.; Sahoo, S.K. Nanotechnology-based combinational drug delivery: An emerging approach for cancer therapy. Drug Discov. Today 2012, 17, 1044–1052.
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr. Metab. Insights 2016, 9, 25–30.
- Hu, J.; Wang, J.; Wang, G.; Yao, Z.; Dang, X. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int. J. Mol. Med. 2016, 37, 690–702.
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528.
- Voinea, M.; Simionescu, M. Designing of “intelligent” liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002, 6, 465–474.
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent Design to Treat Complex Disease. Pharm. Res. 2006, 23, 1417–1450.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966.
- Iyer, A.K.; Greish, K.; Seki, T.; Okazaki, S.; Fang, J.; Takeshita, K.; Maeda, H. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J. Drug Target. 2007, 15, 496–506.
- Shin, H.-C.; Alani, A.W.G.; Rao, D.A.; Rockich, N.C.; Kwon, G.S. Multi-drug loaded polymeric micelles for simultaneous delivery of poorly soluble anticancer drugs. J. Control. Release 2009, 140, 294–300.
- Kenmotsu, H.; Yasunaga, M.; Goto, K.; Nagano, T.; Kuroda, J.; Koga, Y.; Takahashi, A.; Nishiwaki, Y.; Matsumura, Y. The antitumor activity of NK012, an SN-38-incorporating micelle, in combination with bevacizumab against lung cancer xenografts. Cancer 2010, 116, 4597–4604.
