Research article
Vol.8 Issue.1 Page No 8-15
Suman Sharma1, Sharmila Pokharna2, Hariom Nagar1*
1Suresh Gyan Vihar University, Jaipur , Rajasthan, India
2LBS (PG) College, Jaipur, Rajasthan, India.
Keywords
Corrosion inhibition,
Aluminium,
Weight loss,
prosopis cineraria,
sodium hydroxide
*Corresponding author’s e-mail addresses- hariom.nagar@mygyanvihar.com,
Paper Received- 16/09/2021 Paper Published – 09/02/2022
Abstract
The inhibition efficiency of prosopis cineraria sangria pod extract has been evaluated on aluminium in NaOH solutions by weight loss methods. The values of inhibition efficiency that are obtained showed its dependency upon the temperature and concentration of inhibitor. Maximum inhibition efficiency was found to be at 50 ppm of extract concentration of prosopis cineraria sangri pod extract. With increase in inhibitor concentration the inhibition efficiency also increased but decreases with increasing temperature. The inhibitor was environmentally safe for aluminium corrosion in the acidic medium and low cost too. The prosopis cineraria sangri pod extracts contains alkaloids, saponin, tannins, aspartic acid, glutamic acid, serine, glycine, histidine, threonine, arginine, alanine, proline, tyrosine, valine, methionine, cysteine, isoleucine, leucine, lysine and phenylalanine are responsible for inhibitory action. Prosopis cineraria pod extract is better corrosion inhibitor in 1M sodium hydroxide solution.
- Introduction
Lot of metals is used in different industries but aluminum and its alloy has a vast applications in industries because of its use in corrosive environment and in aggressive media. Aluminium is used in coating various reaction vessels, reaction tanks and pipes etc. for industrial uses because of its light weight, availability and low cost (Bouklah and Hammouti 2006). However in aggressive media, if the metal is not protected than surface sites gets dissolved in aggressive media and causes severe loss and malfunctioning of industrial equipment. Therefore, the best way is using inhibitor to protect the metal surface from corrosion (Okafor et al. 2005). Those organic compounds has been found best for this purpose that contain N, S or O and their inhibiting action is widely attributed to their adsorption onto the metal/solution interface (Oguzie 2005). Using organic or inorganic inhibitors now-days is an environmental threat so, it has become a necessity to restrict its use and has to be replaced by green inhibitors. A lot of literatures shows that many green inhibitors are effective inhibitors for metals in aggressive media like acacia seyal-var seyal (Buchweishaija and Mhinzi 2008), Prosopis spicigera leaves ( Jewers et al. 1976), pantagonian prosopis species (Mazzuca and Balzaratti 2003), Aloe plant extract (Al-Turkustani et al. 2010) and coffee husk (Fouda et al. 2015) etc. The present study, shows use of such green inhibitor i.e. prosopis cineraria pod extract, has been used to inhibit the corrosion of aluminium caused by acids.
Prosopis cineraria plant is commonly found in dry and arid regions of north western India, southern India, Pakistan, Afganishtan and Arabia. Many delicious dried vegetables, curries and pickles are prepared from dried green sangria pods. Sangri contains alkaloids, saponin, tannins, aspartic acid, glutamic acid, serine, glycine, histidine, threonine, arginine, alanine, proline, tyrosine, valine, methionine, cysteine, isoleucine, leucine, lysine and phenyalanine (Abdel-Gaber et al. 2008; Oguzie 2005).
- Materials and method
2.1. Preparation of extract prosopis cineraria sangri pods
Extract of prosopis cineraria sangri pods in the present study was prepared as per literature reports (Sharma et al. 2019). For preparation of extract fine powder of sangria pods was grinded from air and shade dried sangria pods. This powdered dried pod was soaked in sufficient amount of distilled ethyl alcohol in 500ml round bottom flask. When the soaking period was completed, the ethanolic solution was refluxed, thereafter, distilled to concentrate the inhibiting chemicals and finally it is filtered to remove if any suspended impurities are present. The mass of plant extract was dried and was used for present experiment as corrosion inhibitor.
2.2. Preparation of specimen
Aluminium sheets (3 mm thick) with normal composition 0.8% Si, 0.7% Fe, 0.4% Cu, 1.2% Mg, 0.35% Cr, 0.25% Zn, 0.15% Ti and the rest aluminium was taken for experiments. Rectangular coupons of the sheet were cut into the size of 1.0×4.0×0.2 cm. The surface of the coupons were polished with emery paper thoroughly, from lower grade- 150 to water, then acetone was used for degreasing, and desiccated overnight after wrapping in filter paper and after that weighing was done (Sharma et al. 2019).
2.3. Mass loss method
As per prescribed in literature by Wang et al. (2011) weight loss measurements were conducted in the present study under controlled condition having temperature maintained at 303-333 k in thermostat water bath in a beaker with 200ml test solution. The aluminium coupons were suspended in the beaker after weighing with the help of rod and hook. The coupons were kept in flask with different inhibitor concentration at 303 K, then washed thoroughly with distilled water, and wrapped with filter paper and desiccated overnight. After drying overnight the final weights of the coupons were than obtained. The weight loss, in grains, was calculated by the difference in the weight of the aluminium coupons before and after immersion in different test solutions determined using digital balance with sensitivity of ±0.1 mg. Then, at different temperature the test were repeated. The tests were carried out in set of three so as to get good and reliable results.
The equation below was used to calculate weight loss:
∆W=W1-W2 (1)
Where
W1= Initial weight of coupons before immersion
W2= Final weight of coupons after immersion
∆W= Weight loss of coupons.
Than with the help of the resulting weight loss data, the percentage inhibition efficiency (%IE) was calculated as follows:
“%I.E. = [1-WLi/WLb] ×100” (2)
WLi= Weight loss of coupons in inhibited solution.
WLb= Weight loss of coupons in blank/uninhibited solution.
- RESULT AND DISCUSSION
3.1. Effect of inhibitor concentration
Weight loss measurement was used to calculate the corrosion rate and inhibition efficiency of the inhibitor in sodium hydroxide. The prosopis cineraria pod extract concentration showed effects on weight loss in 1M NaOH are shown below in figure 1. As the concentration of inhibitor was increased it showed a decrease in the weight loss and the rate of corrosion up to an optimum level after which there was not any significant change in rate of corrosion and inhibition efficiency when concentration of inhibitor was increased. Maximum efficiency showed by the inhibitor in NaOH is 83.86% at an optimum concentration of 50ppm. Further increase in extract concentration did not cause any significant change in the inhibition efficiency (Sharma et al. 2019). The values of percentage efficiency in corrosion rate obtained from weight loss method at different concentration of pod extract the values of corrosion rate and percentage efficiency at 303 K were obtained from weight loss method are shown in figure below.
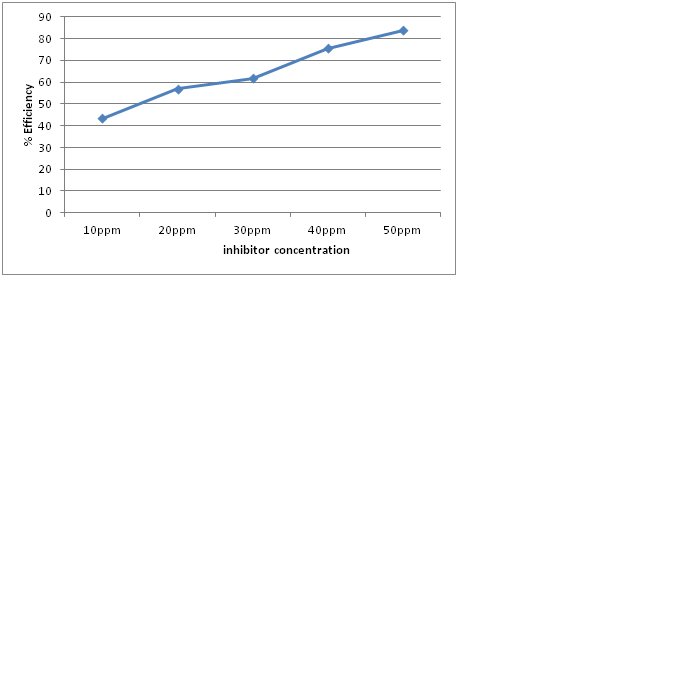
Figure 1: Variation in efficiency with different concentration of inhibitor
3.2. Effect of temperature
Different weight loss measurements were carried out in the temperature range of 303-333 K to calculate the stability of adsorbed layer/film of the inhibitor on the surface of aluminum metal in the corrosion process in the acidic medium in the presence of prosodies cineraria pod extract at optimum concentration. The figure 2 shows the results that were obtained from the observation. The figure 2 shows the evidence that with the increasing temperature the inhibition efficiency decreases. This is happening because of the increased in rate of dissolution process of aluminum and partial desorption of the inhibitor from the metal surface with temperature.
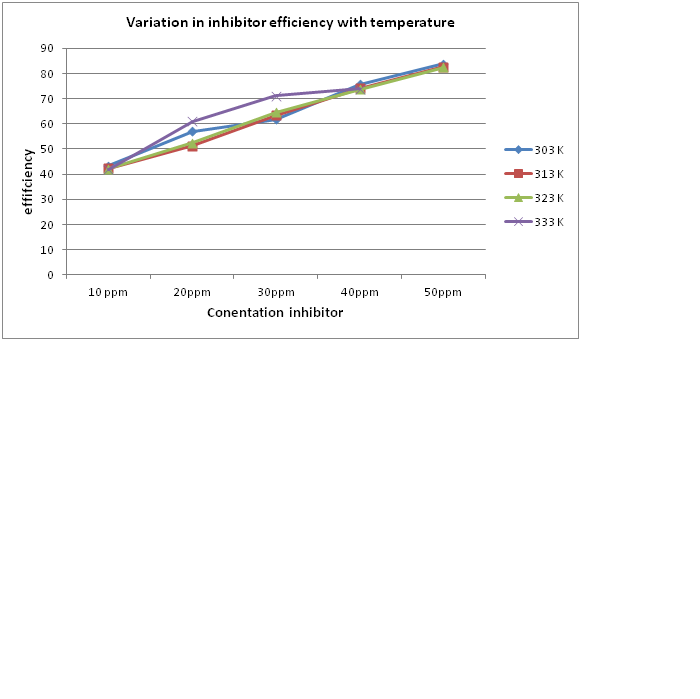
Figure 2: Variation in inhibitor efficiency at different temperature
- CONCLUSIONS
Following conclusions can be made from the observations of this experiment; a) it was found that the extract of prosopis cineraria pod to be an effective inhibitor for the corrosion inhibition of aluminium in 1M NaOH at a temperature range 303-333 K, b) the inhibition efficiency reached to a maximum of 83.86% at a concentration as low as 50ppm of the extract. This was calculated from gravimetric measurements, c) with the increased concentration of extract the inhibition efficiency increased to an optimum concentration of extract, and d) the inhibition efficiency decreased with increasing temperature.
ACKNOWLEDGEMENTS
The authors are grateful to the anonymous referees for their useful suggestion. Thanks are due to the Department of Applied Science, Suresh Gyan Vihar University, Jaipur for providing necessary facilities and support.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES
- Abdel-Gaber, A. M., E. Khamis, H. Abo-ElDahab, and S. H. Adeel. 2008. Inhibition of aluminum corrosion in alkaline solutions using natural compound. Materials Chemistry and Physics 109: 297-305.
- Ajanaku, K. O., C. O. Ajanaku, A. A. Akinsiku, A. Falomo, A. Edobor-Osoh, and O. M. John. 2012. Eco-friendly impact of vernonia amygdalina as corrosion inhibitor of aluminium in acidic media. Chemistry Journal 2: 153-157.
- Al-Turkustani, A. M., S. T. Arab, and R. H. Al-Dahiri. 2010. Aloe plant extract as environmentally friendly inhibitor on the corrosion of aluminium in Hydrochloric acid in absence and presence of Iodide ions. Modern Applied Science 4: 105-124.
- Bouklah, M., and B. Hammouti. 2006. Thermodynamic characterization of steel corrosion for the corrosion inhibition of steel in sulphuric acid by Artimisia. Portugaliae Electrochimica Acta 24: 457-468.
- Buchweishaija, I., and Mhinzi. 2008. “Natural products as a source of environmentally friendly corrosion inhibitors: The case of gum exudates from Acacia seyal var. Seya. Portugaliae Electrochimica Acta 26: 257-265.
- Fouda, A. S., H. S. Gadow, and K. Shalabi. 2015. Chemical and electrochemical investigations of coffee husk as green corrosion inhibitor for aluminium in hydrochloric acid solution. International Journal of Recent Research and Applied studies 23: 28-45.
- Jewers, K., M. J. Nagler, K. A. Zirvi, F. Amir, and F. H. Cottee. 1976. Lipids, sterols, and a piperidine alkaloid from Prosopis Spicigera Phytochemistry 15: 238-240.
- Mazzuca, M., and V. T. Balzaratti. 2003. Fatty acids, sterols and other steroids from seeds of pantagonian prosopis Journal of the Science of Food and Agriculture 83: 1072-1075.
- Oguzie, E. E. 2005. Inhibition of Acid Corrosion of Mild Steel by Telfariaoccidentalis Pigment & Resin Technology 34: 321-326.
- Okafor, P. C., U. J. Ekpe, E. E. Ebenso, E. M. Umoren, and K. E. Leizou. 2005. Inhibition of mild steel corrosion in acidic medium by Allium sativum extraxts. Bulletin of Electrochemistry 21: 347-352.
- Sharma, S., S. Pokharna, and H. Nagar. 2019. Comparative study of corrosion inhibition of aluminium in HCl and H2SO4 media by prosopis cineraria. International Journal of Research and Analytical Reviews 6: 873-875.
- Wang, F., C. Gao, K. Kuklane and I. Holmér. 2011. Determination of Clothing Evaporative Resistance on a Sweating Thermal Manikin in an Isothermal Condition: Heat Loss Method or Mass Loss Method?. The Annals of Occupational
