Volume 6, Issue 1, 2020, pp. 7-13
Shalini Dixit1,2, Gaurav Kumar Sharma1, Tarun Patodia1,2, Balram Tripathi2
1Department of Physics, Suresh Gyan Vihar University, Jaipur
2Department of Physics, S. S. Jain Subodh PG (Autonomous) College, Jaipur
E-mail: shaliniytiwari@gmail.com , balramtripathi1181@gmail.com
Abstract
It is well known that three challenges of hydrogen economy, that is, production, storage, and transportation put tremendous stress on scientific community for the past several decades. Based on several investigations, reported in literature, it is observed that the storage of hydrogen in solid form is more suitable option to overcome the challenges like its storage and transportation. In this form, hydrogen can be stored by absorption (metal hydrides and complex hydrides) and adsorption (carbon materials). Compared to absorption, adsorption of hydrogen on carbon materials is observed to be more favorable in terms of storage capacity. Taking in to account of these facts, in this short review, an overview on hydrogen adsorption on Nickel intercalated carbon nanotubes is presented. Synthesis processes of carbon nanotubes is discussed in brief along with their hydrogen storage capacities at different operating conditions, and thermodynamic properties and reaction kinetics. In addition, different methods to improve hydrogen storage capacities of carbon nanotubes are presented in detail. Finally, comparison is made between carbon nanotubes and Nickel intercalated carbon nanotubes to estimate the amount of hydrogen that can be stored and retract practically.
- Introduction
Renewable energy sources are becoming increasingly wide spread because of increasing energy consumption, decreasing fossil-based energy reserves, and intensifying environmental problems due to the use of fossil-based energy resources [1]. Production of hydrogen energy has been increased in recent years because hydrogen energy does not harm the environment and can be produced in a sustainable way [2]. The most important characteristics of hydrogen energy, namely the characteristics that distinguish it from other alternative energy sources, are its capacity to be widely produced, its low weight and energy density. Although many production methods exist for hydrogen energy, storage methods are still limited and problematic [3]. Adsorbent materials have frequently been used in the storage of hydrogen energy in recent years to overcome these problems and the best performing of these adsorbent materials are carbon nanotubes [4,5]. Carbon nanotubes store hydrogen up to 14 wt % due to their mechanical and electrical properties, large surface areas and abundant pore volume [6]. There are single walled and multi walled carbon nanotubes produced in general, however producing a high volume of sin- gle walled carbon nanotubes is difficult, multi-walled carbon nanotubes (MWCNTs) are preferred to single-walled carbon nanotubes in hydrogen storage [7–9].
Dillon et al. claimed that Single walled carbon nanotubes (SWCNTs) adsorb hydrogen between 5 and 10 wt % inside the pores of molecular dimensions [10]. This result led to intense interest in carbon nanotubes for hydrogen adsorption. How- ever, Hirscher et al. was measured SWCNTs hydrogen adsorption capacity up to 0.01 wt % by thermal desorption spectroscopy at ambient pressures and temperature. They also noted that carbon nanotubes must be used in high quantities when storing hydrogen, otherwise the amount of stored hydrogen cannot be accurately measured [11]. More- over, Zhu et al. found hydrogen adsorption capacity to be 5 wt% in MWCNTS at 300 K under 10 MPa pressure [12]. However, Tibbetts et al. found that SWCNTs and MWCNTs uptake hydrogen less than 0.1 wt % under a pressure of 3.5 MPa and at room temperature [13]. The differences in the results of the studies of the carbon nanotubes storage may be due to the weakness of the Wander Waals bonds which is established between hydrogen carbon nanotubes. The adsorbent capacity, and hence the hydrogen storage capacity of MWCNTs can be increased by adding various metals. Adding various metals and chemical compounds to the nanotubes increases the porosity, the dipole-dipole interaction, and thereby enables higher hydrogen storage capacity [14]. Numerous studies have demonstrated that hydrogen uptake capacity is enhanced by doping metals and chemical compounds such as Ni, Pd, Pt, Li, TiO2, Al2O3, ZnO, BN [15–17]. The doped nanotubes are used also for many highly demanding applications such as lead removal, heavy metal removal, energy storage, biosensors, agriculture, field- emitting devices etc. [18–21].
Furthermore, Zacharia et al. claimed that metal nanoparticles may increase hydrogen uptake approximately by 30% on carbon nanotubes via spill over mechanism. It was cited that the capacity of hydrogen adsorption on Pd & V doped MWCNTs was measured 0.69 wt % at 2 MPa by using Sievert’s like apparatus [22]. Rather et al. indicated that TiO2 doped carbon nanotubes adsorbed 0.40 wt % of hydrogen under pressure of 18 bar, also this result exhibited this nanotubes hydrogen adsorption capacity five times higher than purified CNTs. Studies conducted so far have shown that the surface modification of carbon nanotubes is done via metals and chemical compounds which is responsible for high amount of hydrogen storage [22–24].
The hydrogen uptake capacity of Ni-MWCNTs was studied by Kim et al. and founded that Ni nanoparticles are spilled over on MWCNTs by incipient wetness impregnation method and the spreading of Ni nanoparticles over MWCNTs allows the hydrogen to make chemical bonds on the surface of MWCNTs. Additionally, they showed that 2.8% of Ni-MWCNTs deposited hydrogen approximately 1.2 wt % at ambient tem- perature [24].
Reyhani et al. observed that Ni nanoparticles display the maximum hydrogen adsorption capacity of 1.54 wt % at room temperature and atmospheric pressure. It was concluded that hydrogen molecules dissociate on metal particles and hydrogen is stored between the walled of nanotubes due to diffusion [25]. However, because of the inconsistency of the level of hydrogen storage in these studies, we were eager to work on this field.
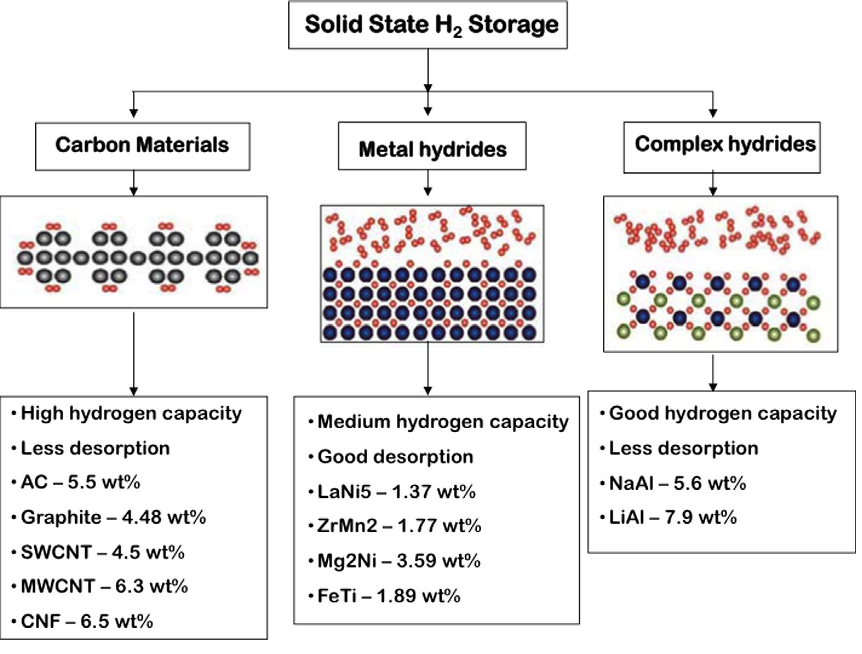
Fig.1 Different solid-state hydrogen storage options [26]
- Experimental :
2.1 Materials
MWCNTs of 10-20 nm in width and less than 2 µm length (99.99% purity) N, N-Dime- thylformamide (DMF) (99% purity), sulphuric acid (H2SO4) (99% purity), Ni, and nitric acid (HNO3) (65% purity) were purchased from Sigma Aldrich.
2.2 Preparation of Ni-doped MWCNTs
150 mg MWCNTs were placed in a solution of 1 M of Ni(NO3)26H2O and subjected to ultrasonification to prepare Ni-MWCNTs nanocomposites. The nanotubes were intercalated with Ni nanoparticles by an ultrasonicator for 1 h. Subsequently, the mixture was dispersed by using DMF using ultrasonicator for 3 h. Thereafter, the nanotubes were kept in an oven at a temperature of 200 ○C for 2 h to remove any impurities.
2.3 Sample characterization
The Zeiss Ultra Plus field emission scanning electron microscopy (FESEM), FEI 200S transition electron microscopy (TEM), Rigaku Ultima IV X-ray diffraction (XRD) using CuKα radiation have been used to investigate the morphology of the synthesized Ni-MWCNTs. Ni-MWCNTs elemental composition investigated by elemental diffraction Bruker X- ray spectroscopy (EDX).
2.4 Hydrogenation studies
Hydrogen uptake capacity of Ni-MWCNTs was measured gravimetrically at different pressures (4, 8, 12, 16, 20 bar) using a Sievert’s-like apparatus. 150 mg of Ni-doped MWCNTs were placed in the storage tank. To remove impurities and possible moisture, 10-2 torr vacuum was applied for 3 h at room temperature. Thereafter, the prepared nanocomposites interacted with hydrogen for 45 min at a flow rate of ~0.5 L/ min, under 4 bar pressures (Fig. 1).
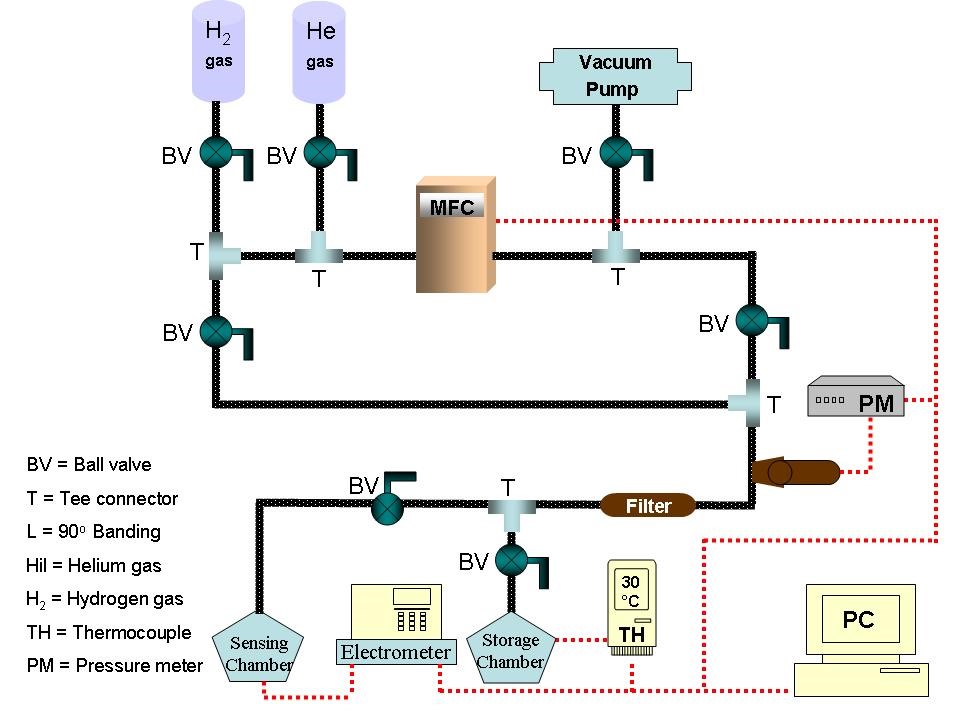
Fig.2 Block diagram for Sievert’s type hydrogen storage setup
- Hydrogen storage mechanism & Review Results
- 1.Physisorption:
The phenomenon is based on the van der Waals interaction between carbon materials and hydrogen molecules. The interaction energy between solid material and hydrogen mol- ecules can be estimated by
E= αH2 αsubstrate
R6
where α is the polarizability and R is the interaction distance.
Here, the polarizability of hydrogen is fixed; therefore, the only way to increase the interaction energy is the selection of high polarizable materials. Average interaction energy ranges from 4 to 5 kJ mol−1 that shows a weak interaction between hydrogen molecules and carbon materials. As a result, increasing temperature can desorb hydrogen consequently less storage capacity can be observed at higher temperatures.
It is assumed that the minimum surface area required for the adsorption of 1 mol of hydrogen may be 85.917 m2 mol−1. Also, maximum hydrogen storage capacity of 3 wt% was reported for graphite based on the surface area of 1315 m2 mol−1 of a single graphene sheet. In this way, the theoretical hydrogen storage capacity (wt%) of a carbon material can be calculated by multiplication of SSA with 2.27 × 10−3. [27] Based on this approximation, a SSA of around 3300 m2 g−1 will be needed to achieve the UFF US Department of Energy (US DOE) target of 7.5 wt%.
3.2.Chemisorption:
Every carbon atom can be utilized as interaction site for chemisorption, if the covalent chemical bonding between carbon atoms is effectively used. According to first principle calculation, it is reported that on CNTs, a dissociative chemisorption of hydrogen is feasible on carbon materials. At high pressure, the hydrogen molecules break into atoms facilitating the formation of two C H bonds that result in shortening of distance between two adjacent tubes which leads in dissociative adsorption of hydrogen. Here, also, desorption takes place at higher temperatures and is not very useful for practical hydrogen storage. In 2006, Li et al. [28] have suggested that adsorption of hydrogen in carbon materials occurs with two different mechanisms. In first mechanism, hydrogen molecules get adsorbed on the surface of carbon materials. As per the sec- ond mechanism, hydrogen molecules penetrate into interlayer space. The study showed that the higher hydrogen adsorption occurs through first mechanism where the SSA plays very important role, which concluded that high hydro- gen storage capacity could be achieved by high surface area.
Songu L Kaskun et [29] reported various results for nickel doped multiwalled carbon nanotubes and find out that maximum hydrogen adsorption capacity was measured to be 0.298 wt % under the pressure of 20 bar. Which reveals that adsorption phenomenon in Ni-MWCNTs was physical. The experimental report by Chen et al.[30] showed that CNT doped with alkali metals could adsorb up to 20 wt% of hydrogen at 653 K and 10 bar. However, it was later suggested that the presence of water might have influenced this result. Yang[31] revisited these data by preparing the doped nanotubes follow- ing the same procedure and measured the adsorption/desorption using a comparable thermogravimetric analyzer. Moisture drastically increased the weight gain and led to erroneous results; however in dry hydrogen, adsorption of only 2.5 wt% for lithium-doped nanotubes and 1.8 wt% for potassium-doped nanotubes were observed and these results were independently confirmed by Pinkerton et al.[32].These observations are also confirmed further by the investigation of Hirscher et al.[33].
SWNTs and MWNTs were extensively investigated for their hydrogen storage capacity upon their discovery and it was observed that at room temperature, aligned and open MWNTs possess hydrogen storage capacity of 1.97 wt% at ambient temperature,[34] 4 wt% for synthesized by the floating catalyst method[35] and 6.3 wt% [36] at 4 MPa, 10 MPa, and 14.8 MPa, respectively.
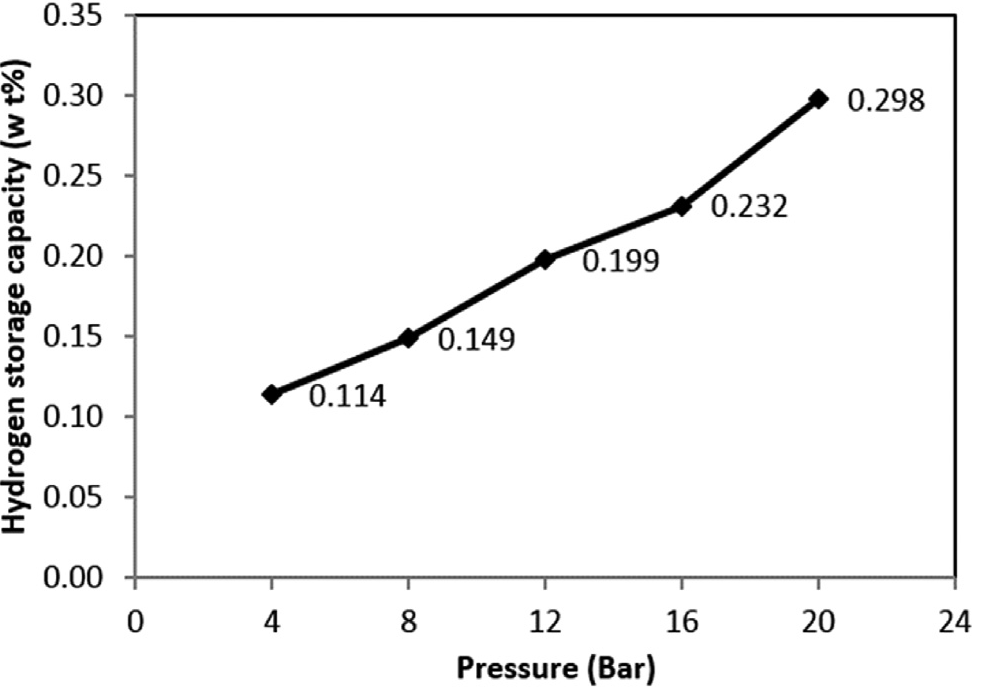
Fig.3. Hydrogen storage in Ni-MWCNTs [29]
The hydrogen storage capacities of CNTs are mainly depend on its structure, pretreatments, geometry, structural defects, operating pressure, temperature, and so on. The possible hydro- gen storage site is inside the tube, outside the tube, between tubes (bundles and ropes), and between shells (in case of MWCNT). One more advantage with CNTs is its known carbon structure. This aspect helps correlating experimental data with theoretical predictions.
- Conclusion
In summary, the influence of Ni doping on MWCNTs and the effect of pressure on Ni-MWCNTs for the hydrogen storage were reviewed. The maximum uptake at 20 bar pressure was 0.298 wt % . The results show that, the amount of stored hydrogen increased as the pressure is increased. Ultimately, the addition of Ni nanoparticles on MWCNTs have enhanced hydrogen storage capacity under moderate pressures and Ni- MWCNTs can be considered as an alternative Hydrogen storage medium.
References
- International Energy Agency (IEA). World energy outlook. 2017 (also available at World Energy Outlook 2017, accessed in December 2017).
- Acar C, Dincer I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int J Hydrogen Energy 2014;39:1–12.
- Zhou Progress and problems in hydrogen storage methods. Renew Sustain Energy Rev 2005;9(4):395–408.
- Cao A, Zhu H, Zhang X, Li X, Ruan D, Xu C, et al. Hydrogen storage of dense-aligned carbon nanotubes. Chem Phys Lett 2001;342(5–6):5104.
- Dresselhaus MS, Dresselhaus G, Avouris P. Carbon nanotubes: synthesis, properties and application. Berlin: Springer-Verlag; 2001.
- Lee SM, Lee YH. Hydrogen storage in single-walled carbon Appl Phys Lett 2000;76(20):2877–9.
- Darkrim FL, Malbrunot P, Tartaglia Review of hydrogen storage by adsorption in carbon nanotubes. Int J Hydrogen Energy 2002;27:193–202.
- Yuan J, Zhu Y, Li Y, Zhang L, Li Effect of multi-wall carbon nanotubes supported palladium addition on hydrogen storage properties of magnesium hydride. Int J Hydrogen Energy 2014;39:10184–94.
- Chambers A, Park C, Baker RTK, Rodriguez NM. Hydrogen storage in graphite nanofibers. J Phys Chem B 1998;102: 4253–6.
- Dillon A, Jones KM, Bekkedahl TA, Kiang Storage of hydrogen in single-walled carbon nanotubes. Nature 1997;386:377.
- Hirscher M, Becher M, Haluska M, Zeppelin F, Chen X, Weglikowska UD. Are carbon nanostructures an efficient hydrogen storage medium. J Alloy Comp 2003;356–357:433–7.
- Zhu HW, Ci LJ, Chen A, Mao ZQ, Xu CL, Xiao X, et al. Hydrogen uptake in multi-walled carbon nanotubes at room temperature. Int. Journal Hydrogen Energy 2000;286:560–4.
- Tibbetts GG, Meisner GP, Olk Hydrogen storage capacity of carbon nanotubes, filaments, and vapor-grown fibers. Carbon 2001;39:2291–301.
- Wu H, Wexler D, Ranjbartoreh AR, Liu H, Wang Chemical processing of double-walled carbon nanotubes for enhanced hydrogen storage. Int J Hydrogen Energy 2010;35:6345–9.
- Wu HB, Chen P, Lin J, Tan Hydrogen uptake by carbon nanotubes. Int J Hydrogen Energy 2000;25:261–5.
- Jiang J, Gao Q, Zheng Z, Xia K, Hu J. Enhanced room temperature hydrogen storage capacity of hollow nitrogen- containing carbon spheres. Int J Hydrogen Energy 2010;35:210–6.
- Vaisman L, Marom G, Wagner HD. Dispersions of surface modified carbon nanotubes in water-soluble and water in soluble polymers. Adv Funct Mater 2006;16:357–63.
- Gupta VK, Agarwal S, Saleh TA. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 2011;185:17–23.
- Anastopoulos I, Anagnostopoulos VA, Bhatnagar A, Mitropoulos AC, Kyzas A review for chromium removal by carbon nanotubes. Chem Ecol 2017;33:572–8.
- [20] Haruna K, Saleh TA, Hossain MK, Al-Saadi AA. Hydroxylamine reduced silver colloid for naphthalene and phenanthrene detection using surface-enhanced Raman spectroscopy. Chem Eng J 2016;304:141–8.
- Saleh TA, Sarı A, Tuzen M. Effective adsorption of antimony (III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem Eng J 2017;307:230–8.
- Zacharia R, Kim KY, Kibria AKMF, Nahm Enhancement of hydrogen storage capacity of carbon nanotubes via spill-over from vanadium and palladium nanoparticles. Chem Phys Lett 2005;412:369–75.
- Rather SU, Mehraj-ud-din N, Zacharia R, Hwang SW, Kim AR, Nahm KS. Hydrogen storage of nanostructured TiO2– impregnated carbon nanotubes. Int J Hydrogen Energy 2009;34(2):961–9.
- Kim HS, Lee H, Han K-S, Kim J-H, Song M-S, Park M-S, et Hydrogen storage in Ni nanoparticle-dispersed multiwalled carbon nanotubes. Phys Chem B 2005;109:8983–6.
- Reyhani A, Mortazavi SZ, Mirershadi S, Moshfegh AZ, Parvin P, Golikand AN. Hydrogen storage in decorated multiwalled carbon nanotubes by Ca, Co, Fe, Ni, and Pd nanoparticles under ambient conditions. J Phys Chem 2011;115:6994–7001.
- Man Mohan, Vinod Kumar Sharma, Anil Kumar, V. Gaythri, Hydrogen storage in carbon materials- A review, Energy Storage 2019 (1-35) Jhn Wiley & Sons, https://doi.org/10.1002/est2.35.
- Strobel R, Garche J, Moseley PT, Jorissen L, Wolf Hydrogen storage by carbon materials. J Power Sources. 2006;159: 781-801.
- Li Y, Zhao D, Wang Y, Xue R, Shen Z, Li X. The mechanism of hydrogen storage in carbon materials. Int J Hydrogen Energy. 2006;32:2513-2517.
- Songu¨l Kaskun, Muhammet Kayfeci , The synthesized nickel doped multiwalled carbon nanotubes for hydrogen storage under moderate pressures, International Journal of Hydrogen Energy (2018), https://doi.org/10.1016/j.ijhydene.2018.01.084.
- Chen P, Wu X, Lin J, Tan KL. High H2 uptake by alkali-doped carbon nanotubes under ambient pressure and moderate tempera- tures. Science. 1999;285:91-93.
- Yang Hydrogen storage by alkali-doped carbon nanotubes revisited. Carbon. 2000;38:623-641.
- Pinkerton FE, Wicke BG, Olk CH, et Thermogravimetric measurement of hydrogen absorption in alkali-modified carbon materials. J. Phy. Chem. B. 2000;104:9460-9467.
- Hirscher M, Becher M, Haluska M, et al. Hydrogen storage in sonicated carbon materials. Appl Phys Mater Sci Process. 2001; 72:129-132.
- Lee H, Kang YS, Kim SH, Lee JY. Hydrogen desorption proper- ties of multiwall carbon nanotubes with closed and open struc- tures. Appl Phys Lett. 2002;80:577-579.
- Li X, Zhu H, Ci L, et al. Hydrogen uptake by graphitized multi- walled carbon nanotubes under moderate pressure and at room Carbon. 2002;39:2077-2088.
- Hou P, Yang Q, Bai S, Xu S, Liu M, Cheng H. Bulk storage capacity of hydrogen in purified multiwalled carbon nanotubes. J Phys Chem B. 2002;106:963-966.
