Review article
Vol. 7 Issue 2 Page No. 35-44
Akash Garg*and Ankur Jain
School of Applied Sciences, Suresh Gyan Vihar University, Jaipur, India
*Corresponding author’s e-mail addresses: akashgarg832@gmail.com
Received: 09 Feb 2021; Accepted 05 May 2021
Keywords
Metal-organic frameworks
Nanoporous materials
Porosity
Gravimetric capacity
Volumetric capacity
Hydrogen storage
Humidity sensing
Abstract
Metal-organic frameworks (MOFs) for hydrogen storage have continued to receive intense interest over the past several years. MOFs have attracted worldwide attention in the area of hydrogen energy, particularly for hydrogen storage. MOFs are a family of nanoporous materials and class of compounds. MOFs have higher surface area and porosity, low densities, flexible and tuneable porous structure, these are the attractive properties for gas storage. For these reasons MOFs have been extensively studied. In this review, the recent developments of hydrogen storage in MOFs is presented, with a focus on surface area, pore volume, pore size. The review focuses on experimental studies. In this review, an overview on hydrogen adsorption on MOFs are presented. Synthesis processes of MOFs are discussed in brief along with their hydrogen storage capacities at different operating conditions. Different methods to improve hydrogen storage capacities of MOFs are also presented.
Introduction
Hydrogen is a promising vehicular fuel due to its high specific energy, renewability and ability to be produced and oxidized without CO2 emissions. However, due to the low volumetric density of H2 gas, efficient and cost-effective storage of hydrogen remains a challenge (Alauddin Ahmed et al., 2019; Ahmed, A. et al., 2017). To overcome this challenge, storage in solid adsorbents has received significant attention as an alternative to compression in high-pressure tanks (Yang, J., Sudik, A et al., 2010; Siegel, D. J. et al., 2014).
Hydrogen is an ideal energy carrier for a variety of fuel cell applications including mobile, stationary and portable power applications. Hydrogen, a possible complete sustainable solution for rising energy demands and hostile intimidating environmental effects caused by combustion of fossil fuels. Effortless production, high energy density, abundant and zero pollution makes hydrogen most desirable and affordable fuel for onboard applications (Zohuri B. 2019; Abe Jo et al., 2019). Fossil fuels such as petroleum, natural gas, and coal provide more than 80% of all the energy being consumed globally (Rusman NAA et al., 2016; Sun Y et al., 2018; Veziroglu TN et al., 2008; Veziroglu TN 2012; De Jongh PE et al., 2010; Iordache I et al., 2013).
Why study hydrogen storage?
Hydrogen storage is a major enabling technology for the advancement of hydrogen and fuel cell technologies in applications including portable power, stationary power, and transportation. Hydrogen has the highest energy per mass of any fuel; However, its low ambient temperature density results in a low energy per unit volume, hence the need for the development of advanced storage methods with high energy density capability (J.O. Abe et al., 2019).
How hydrogen storage works?
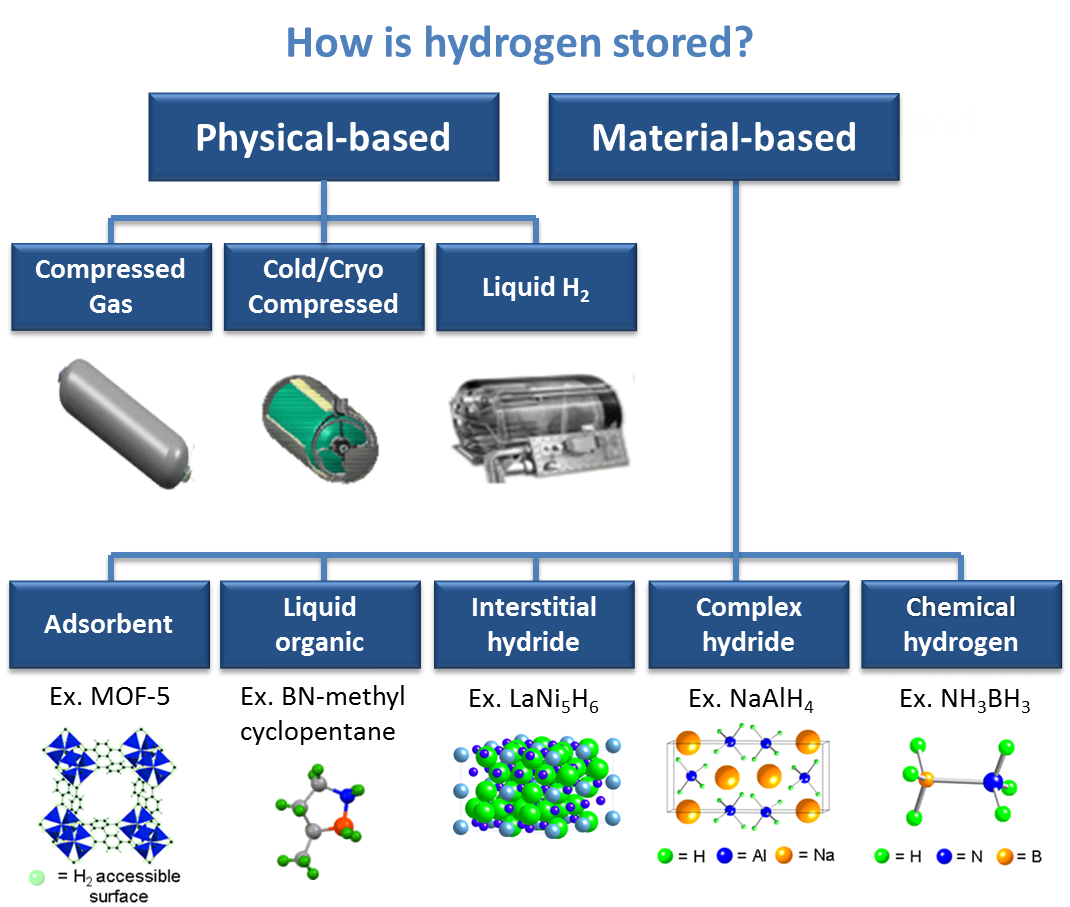
Figure 1: Hydrogen storage (https://hydrogeneurope.eu/hydrogen-storage)
Hydrogen can be physically stored as either a gas or a liquid. Storage of hydrogen in the form of gas usually requires high-pressure tanks (350–700 bar tank pressure). Storage of hydrogen as a liquid requires cryogenic temperatures because the boiling point of hydrogen is −252.8°C at an atmospheric pressure. Hydrogen can also be stored on the surfaces of solids (adsorption) or within solids (absorption) (Vinay Ananthachar et al., 2005; Wong-Foy et al., 2006; Witman, M. et al., 2017). Hydrogen storage is an important challenge for the development and viability of hydrogen-powered vehicles. On-board hydrogen storage in the range of approximately 5–13 kg is required to enable a driving range of more than 300 miles for a full platform of light-duty automotive vehicles using fuel cell power plants (Goldsmith, J. et al., 2013; Rowsell, J.L.C. et al., 2005; Broom, D. P. et al., 2016).
Metal-organic frameworks (MOFs) are probably the most intensively-researched hydrogen adsorbents. Microporous crystalline MOFs are formed by the self-assembly of inorganic metal clusters or ions and organic linkers (Gomez-Gualdron, D. A. et al., 2017; Collins, D. J. et al., 2007; Rosi, N. L. et al., 2003; Bobbitt, N. S., Chen, J. et al., 2016; Colón, Y. J et al., 2017; Batten, S. R. et al., 2013; Sarita Dhaka et al., 2019).
Metal ions + organic linkers = MOFs
MOFs are a family of nanoporous materials. It is a very promising materials for hydrogen storage due to some unique properties such as high surface area, porosity, low density, flexible and tuneable porous structure in comparison to the conventional materials like zeolites (A Dailly et al., 2011; O. Ardelean et al., 2013; R. Balderas-Xicohtencatl et al., 2018). Several highly porous MOFs structures are presented in figure 2.
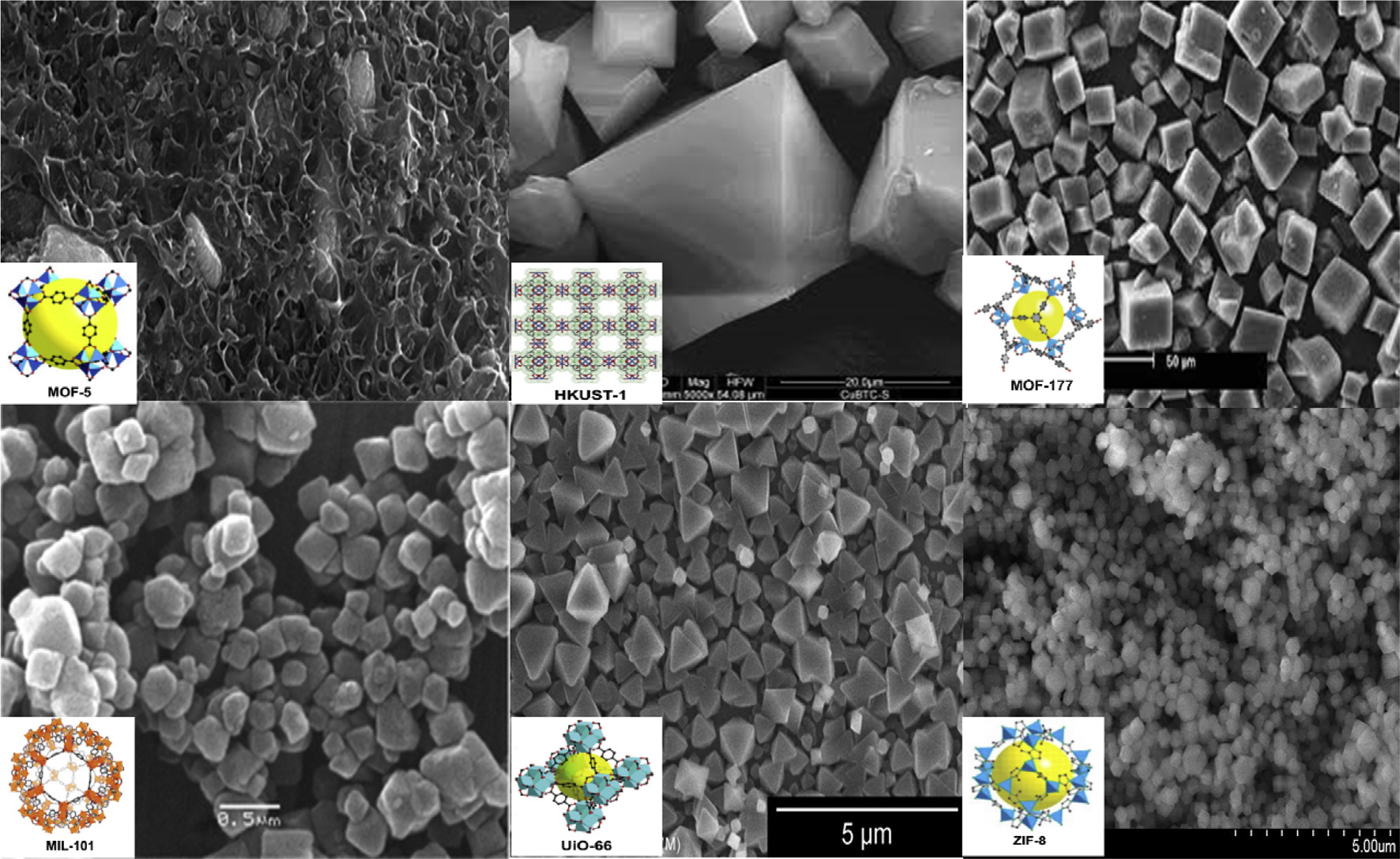
Figure 2: Representative MOFs (Figure was reproduced from Mohadeseh Safaei et al., 2019, with permission from Elesivier).
As a testament to their potential applicability for H2 storage, the MOFs have exceeded the DOE 2021 onboard gravimetric H2 storage technical targets 4.5 wt % at 77 K and 100 bar, however they are not yet to do this at room temperature, for which MOFs is reported to adsorb 1−2 wt % at 100 bar.
Porosity of MOFs
The porosity of MOFs is more than any other nanoporous material, double the record for porous carbon. The surface area of MOF-5 was initially investigated at 2,900 m2/g , but now MOF-5 can be activated to achieve 3,800 m2/g. In such material, 60% is open space, in which gases and organic molecules can be introduced. Differently from other porous materials, MOFs have pores without walls; they are made entirely of struts and intersections. They are open scaffolds, where the struts or intersections are sites for gas molecules to enter. That is the reason they have very high surface area, and this is the optimal way to create high surface area materials. The surface area of MOF-177 was 5500 m2/g. Now, using simple chemistry, we can get 5,500 m2/g. MOFs can be sized for various applications, including catalysis and gas separation (Omar M. Yaghi et al., 2009; R.M. Abhang et al., 2013).
Gravimetric capacity of MOFs
The Gravimetric capacity is the amount of H2 adsorbed per unit mass, expressed as kg H2 kg-1, for example, or as a weight percentage (wt.%), is important because hydrogen has low molar mass. If a storage tank, including the material, weighs too much, the hydrogen fuel cell vehicle’s range will be limited. That is an issue, as long as the driving range, together with very short refueling times, is a major advantage of hydrogen fuel cell vehicles compared to their battery electric counterparts (Thomas CE 2009; Eberle U et al., 2010).
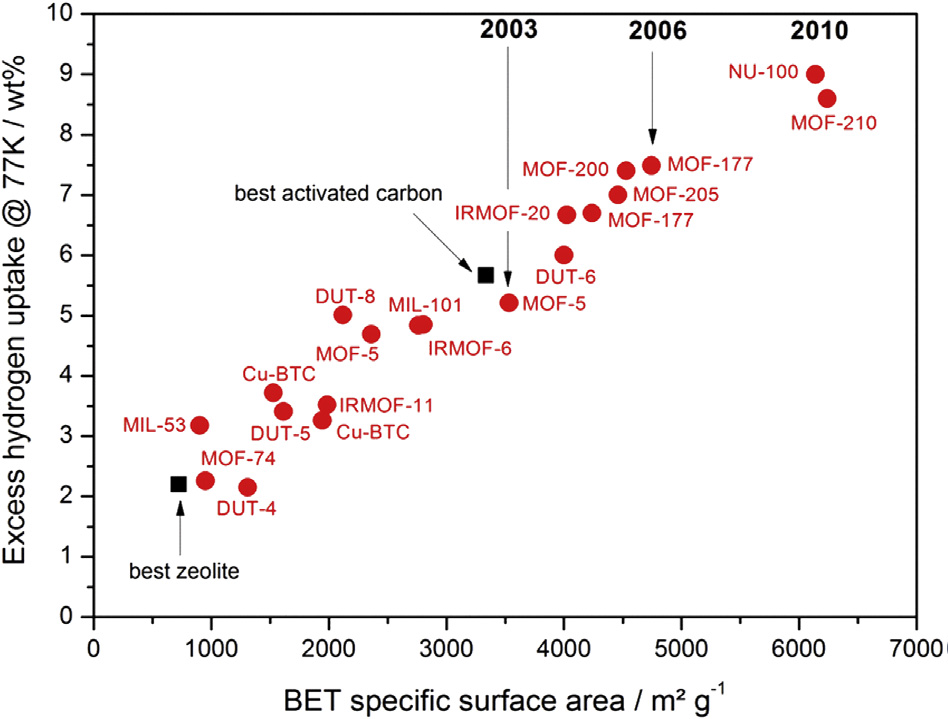
Figure 3: A plot between excess gravimetric H2 uptake, measured at 77 K and 2 MPa or above, for different MOFs, and the best performing zeolite and activated carbon (D.P. Broom et al., 2019). Reproduced, under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Volumetric capacity of MOFs
The Gravimetric capacity determines the weight of the storage tank required to store a given amount of H2. Volumetric capacity, on the other hand, determines the volume of the tank and is defined as, for adsorbents,as the amount of H2 adsorbed per unit volume, for example, as gH2L-1. Calculating the volumetric capacity of a material therefore requires knowledge of its bulk volume or density (Ming Y et al., 2014).The dependence of the absolute volumetric H2 uptake on the volumetric surface area, for different MOFs, is shown in Fig.4.
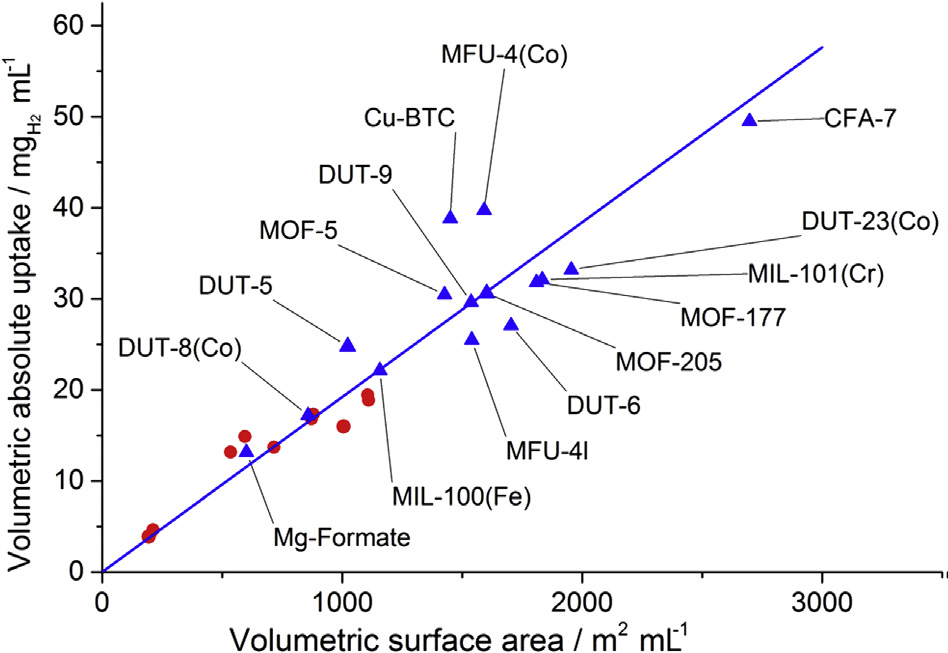
Figure 4: A plot between absolute volumetric H2 uptake and volumetric surface area for various MOFs. Blue triangles show values calculated using single crystal densities and red circles indicate values obtained using packing densities. All measurements were obtained at 77 K, in the pressure range 2-2.5 MPa (D.P. Broom et al., 2019). Reproduced, under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Materials
The first investigation of gas adsorption by a MOF was of methane adsorption in [Cu(4,4-bipy)SiF6]∞, which showed an adsorption capacity comparable to that of zeolites and activated carbons (Noro et al., 2000). The first example of H2 storage in a MOF was investigated in 2003 (Rosi et al., 2003). A wide range of such materials have been reported with increasingly high values for overall storage capacity and with interesting variations observed for the heats of adsorption ∆Hads of H2 depending upon the framework material.
Synthesis
Due to their specific functional and structural properties, MOFs are currently recognized as a considerable group of porous framework. These frameworks are made up of organic ligands or linker and metal ion clusters and metal ions. Without any doubt, another concept significantly involved in the final structure and properties of MOFs is the selected primary building blocks (PBUs). However, many other synthetic methods and parameters, such as pressure, temperature, pH, reaction time and solvent, must be considered as well (Yitong Han et al., 2019). Many different synthetic approaches, including hydrothermal, solvothermal can be applied to produce MOFs relying on the resulting features and structures. The method for the synthesis of MOFs is solvothermal. Usually, metal precursors and organic linkers are dissolved in solvent and placed in a closed reaction vessel for the formation and self-assembly of MOF crystals. The common solvents used include methanol, ethanol, acetonitrile, N,N’-dimethylformamide(DMF), and N,N’-diethylformamide(DEF). The synthesis temperature is generally below from 220°C, and the crystallization time varies from several hours to several tens of days (X.Y. Chen et al., 2005; D. Wang et al., 2010; J.Y. Wu et al., 2013).
Solvothermal Synthesis
Solvothermal synthesis is a method of producing chemical compounds. It is allows for the precise control over the size, shape distribution, and crystallinity of metal oxide nanoparticles or nanostructure products. In 1995, Nalco chemical company and Professor Yaghi reported the synthesis of MOFs by Solvothermal Synthetic. The mixed solution of inorganic salt and organic adding arms is poured into a sealed reaction vessel to heat the mixed solution to form an insoluble framework, and insoluble substances precipitate and form crystals. The temperature of the reactant can reach its boiling point, so the solvent may partially or completely dissolved (H. Li et al., 1998; L. Pineiro-Lopez et al., 2014; S. Qiu et al., 2009).
Hydrothermal synthesis
Hydrothermal synthesis involves the various techniques of crystallizing substances from high-temperature aqueous solutions at high vapour pressures. Most of the investigated MOF materials are synthesized using hydrothermal and solvothermal synthetic conditions, often using sealed autoclaves. Hydrothermal synthesis, where water are used as solvents and solvothermal synthesis, where solvents other than water are used because solvents are with high temperatures and increased pressure. In addition the pure hydro or solvothermal methods, mixtures of water and other solvents also are used to synthesize various MOFs. Hydrothermal techniques plays an important role in preparing strong and stable MOF compounds, compared to the solution reactions (L. Shen et al., 2013; Linjian Zhang et al., 2018).
Conclusion
Currently MOFs have been considered as a new member of nanoporous materials that recently have attracted all the consideration to materials chemistry. It is clear that MOFs will present exceptional advantages compared to other conventional porous materials and it will have a significant impact on the future of porous compounds. While novel structures and materials with notable characteristics have been created through the synthesis of MOF, improvements have been made in this area, expanding its range. This is true for both application area and the building exploited technologies. Arenas of solvothermal and hydrothermal syntheses are just evolving despite widespread use of unoriginal syntheses methods. Typically under mild reaction conditions, these techniques can be use for some materials, which leads to the production of compounds with different features and particles sizes. In particular, it may be considered in the use of MOFs and increase of syntheses. However, these alternate methods have mainly led to the reproduction of popular materials. Therefore, there is no doubt that MOFs will be synthesized in a simple, easy, green and low-cost way. The synthesis of MOFs will be in a large scale to fulfill all of the requirements. The challenges and orientations present the continual expansion interest and a bright future of MOF chemistry.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Dailly A., E. Poirier (2011). Evaluation of an industrial pilot scale densified MOF-177 adsorbent as an on-board hydrogen storage medium. Energy Environ. Sci. 4, 3527.
- Abe JO, Popoola API, Ajenifuja E, Popoola OM (2019). Hydrogen energy, economy and storage: review and recommendation. International Journal of Hydrogen Energy 44, 15072-86.
- Alauddin Ahmed, Saona Seth, Justin Purewal (2019). Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nature Communications. 10,
- Alauddin Ahmed, Yiyang Liu, Justin Purewal, Ly D. Tran, Antek G. Wong-Foy, Mike Veenstra, Adam J. Matzger, Donald J. Siegel (2017). Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ. Sci.10, 2459–2471.
- Batten S. R., Champness N. R., Xiao-Ming Chen, Javier Garcia-Martinez, Susumu Kitagawa , Lars Öhrström , Michael O’Keeffe , Myunghyun Paik Suh and Jan Reedijk (2013). Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 85, 1715–1724.
- Bobbitt, N.S., Chen, J. & Snurr, R.Q. (2016). High-throughput screening of metal–organic frameworks for hydrogen storage at cryogenic temperature. Phys. Chem. C.120, 27328–27341.
- Broom D P, Webb C J, Hurst K E, Parilla P A, Gennett T, Brown C M, Zacharia R, Tylianakis E, Klontzas E, Froudakis G E, Steriotis Th. A, Trikalitis P N (2016). Outlook and challenges for hydrogen storage in nanoporous materials. Phys. A122(3), 151.
- Collins, D. J. & Zhou, H.-C. (2007). Hydrogen storage in metal–organic frameworks. Mater. Chem.17, 3154–3160.
- Colón Y.J., Gómez-Gualdrón, D.A. & Snurr, R.Q. (2017). Topologically guided, automated construction of metal–organic frameworks and their evaluation for energy-related applications. Growth Des.17, 5801–5810.
- Wang, H. He, X. Chen, S. Feng, Y. Niu, D. Sun (2010). A 3D porous metal-organic framework constructed of 1D zigzag and helical chains exhibiting selective anion exchange. CrystEngComm 12, 1041-1043.
- P. Broom, C.J. Webb, G.S. Fanourgakis, G.E. Froudakis, P.N. Trikalitis, M. Hirscher (2019). Concepts for improving hydrogen storage in nanoporous materials. International journal of hydrogen energy 44, 7768-7779.
- De Jongh PE, Adelhelm P. (2010). Nanosizing and nanoconfinement: new strategies towards meeting hydrogen storage goals. ChemSusChem 3, 1332-48.
- Eberle U, von Helmolt R (2010). Sustainable transportation based on electric vehicle concepts: a brief overview. Energy Environ Sci 3(6), 689-99.
- Goldsmith, J., Wong-Foy, A. G., Cafarella, M. J. & Siegel, D. J (2013). Theoretical limits of hydrogen storage in metal–organic frameworks: opportunities and trade-offs. Mater.25, 3373–3382.
- Gomez-Gualdron D.A., Wang T.C., Garcia-Holley P., Snurr R.Q., Hupp J.T., Yildirim T, Farha O.K. (2017).derstanding volumetric and gravimetric hydrogen adsorption trade-off in metal−organic frameworks. ACS Appl. Mater. Interfaces9, 33419–33428.
- Li, C.E. Davis, T.L. Groy, D.G. Kelley, O.M. Yaghi (2017). Coordinatively unsaturated metal centers in the extended porous framework of Zn3(BDC)3. 6CH3OH (BDC= 1, 4-Benzenedicarboxylate). J. Am. Chem. Soc. 120, 2186-2187.
- Iordache I, Gheorghe AV, Iordache M (2013). Towards a hydrogen economy in Romania: statistics, technical and scientific general aspects. International Journal of Hydrogen Energy 38, 12231-40.
- O. Abe, A.P.I. Popoola, E. Ajenifuja, O.M. Popoola (2019). Hydrogen energy, economy and storage: Review and recommendation. International Journal of Hydrogen Energy 44, 15072-86.
- Y. Wu, T.C. Chao, M.S. Zhong (2013). Influence of counteranions on the structural modulation of silveredi (3-pyridylmethyl) amine coordination polymers. Cryst. Growth Des. 13, 2953-2964.
- Pineiro-Lopez, Z. Arcís-Castillo, M.C. Muneoz, J.A. Real (2014). Clathration of fivemembered aromatic rings in the bimetallic spin crossover metal-organic framework [Fe (TPT)2/3{MI (CN)2}2].G(MI=Ag,Au), Cryst. Growth Des. 14, 6311-6319.
- Shen, W. Wu, R. Liang, R. Lin, L. Wu (2013). Highly dispersed palladium nanoparticles anchored on UiO-66 (NH2) metal-organic framework as a reusable and dual functional visible-light-driven photocatalyst, Nanoscale 5, 9374-9382.
- Linjian Zhang, Fangqin Liand, Liangfei Luo (2018). Preparation Methods of Metal Organic Frameworks and Their Capture of CO2. IOP Conference Series: Earth and Environmental Science 108, 042104.
- Ming Y, Purewal J, Liu D, Sudik A, Xu C, Yang J (2014). Thermophysical properties of MOF-5 powders. Microporous Mesoporous Mater 185, 235-44.
- Mohadeseh Safaei, Mohammad Mehdi Foroughi, Nasser Ebrahimpoor, Shohreh Jahani, Ali Omidi, Mehrdad Khatami (2019). A review on metal-organic frameworks: Synthesis and applications. Trends in Analytical Chemistry, 118, 401-425.
- Ardelean, G. Blanita, G. Borodi, M.D. Lazar, I. Misan, I. Coldea, D. Lupu (2013). Volumetric hydrogen adsorption capacity of densified MIL-101 monoliths. Int. J. Hydrog. Energy 38, 7046.
- Omar M. Yaghi, Qiaowei Li (2009). Reticular Chemistry and Metal-Organic Frameworks for Clean Energy. Materials Research Society(MRS) Bulletin 34, 682-690.
- Balderas-Xicohtencatl, M. Schlichtenmayer, M. Hirscher (2018). Volumetric hydrogen storage capacity in metal-organic frameworks. Energy Technol. 6, 578.
- M. Abhang, K.S. Wani, V.S. Patil, S.H. (2013). Perspectives and Challenges of Hydrogen Storage by Metal-Organic Frameworks. Pratibha: International Journal of Science, Spirituality, Business and Technology (IJSSBT), Vol. 2, No.1.
- Rosi N.L., Eckert J., Eddaoudi M., Vodak D.T., Kim J., O’Keeffe M., Yaghi O.M. (2003). Hydrogen storage in microporous metal-organic frameworks. Science300, 1127–1129.
- Rowsell, J. L. C. & Yaghi, O. M (2005). Strategies for hydrogen storage in metal-organic frameworks. Chemie Int. Ed.44, 4670–4679.
- Rusman NAA, Dahari M. (2016). A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. International Journal of Hydrogen Energy 41, 12108-26.
- Qiu, G. Zhu (2009). Molecular engineering for synthesizing novel structures of metaleorganic frameworks with multifunctional properties. Coord. Chem. Rev. 253, 2891-2911.
- Sarita Dhaka, Rahul Kumar, Akash Deep, Mayur B. Kurade, Sang-Woo Ji, Byong-Hun Jeon (2019). Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coordination Chemistry Reviews 380, 330–352.
- Siegel, D. J. & Hardy, B. (2014). Engineering an Adsorbent-Based Hydrogen Storage System: What Have We Learned?(United States Department of Energy, USA).
- Thomas CE (2009). Fuel cell and battery electric vehicles compared. Int J Hydrog Energy 34(15), 6005-20.
- Veziroglu TN (2012). Conversion to hydrogen economy. Energy Procedia 29, 654-6.
- Veziroglu TN, Sahin S. (2008). 21st Century’s energy: hydrogen energy system. Energy Convers Manag 49, 1820-31.
- Vinay Ananthachar, John J. Duffy (2005). Efficiencies of hydrogen storage systems onboard fuel cell vehicles. Solar Energy 78(5), 687-694.
- Witman M, Ling S, Gladysiak A, Kyriakos C, Smit B, Slater B, Maciej H (2017). Rational design of a low-cost, high-performance metal-organic framework for hydrogen storage and carbon capture. Phys. Chem. C.121, 1171–1181.
- Wong-Foy, G., Matzger, A. J. & Yaghi, O. M (2006). Exceptional H2 saturation uptake in microporous metal-organic frameworks. J. Am. Chem. Soc.128, 3494–3495.
- Y. Chen, B. Zhao, W. Shi, J. Xia, P. Cheng, D.Z. Liao, Z.H. Jiang (2005). Microporous metal-organic frameworks built on a ln cluster as a six-connecting node. Chem. Mater. 17, 2866-2874.
- Yahui Sun, Chaoqi Shen, Qiwen Lai, Wei Liu, Da-Wei Wang, Kondo-Francois, Aguey-Zinsou (2018). Tailoring magnesium based materials for hydrogen storage through synthesis: current state of the art. Energy Storage Mater 10, 168-98.
- Yang, J., Sudik A., Wolverton, C. & Siegel, D. J. (2010). High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Soc. Rev.39, 656–675.
- Yitong Han, Hong Yang and Xinwen Guo (2020). Synthesis Methods and Crystallization of MOFs. DOI: 10.5772/intechopen.90435.
- Zohuri B. (2019). Hydrogen energy: challenges and solutions for a cleaner future. Springer; 2019.
