Priyanka A. Patil*1,2, Dr. Preeti Khulbe1
School of Pharmacy, Suresh Gyan Vihar University, Jaipur, Rajasthan 302017, India.
Department of Chemistry, Dr. Shivajirao Kadam College of Pharmacy, Kasabe Digraj, Sangli, Maharashtra, India. 416305.
ABSTRACT
Despite being created for the first time in previous years, triazole pursue to capture the interest of scientists. Due to its numerous biological actions, the triazole is a significant heterocyclic moiety and holds a special place in heterocyclic chemistry. It is a vital thing in the progress of various pharmacological molecules and is available in two isomeric forms: 1,2,4 and 1,2,3-triazole. 1,2,4-triazole offers a broad variety of therapeutically desirable pharmacological possibilities, with pain-relieving, antiseptic, antimicrobial, antioxidant, antiurease, anti-inflammatory, cytotoxic, antiepileptic, and anti-migraine medications. In this review, we have attempted to summarize some of the recent developments in triazole derivative medicinal chemistry, using some instances of their rational mean as anti-cancer, antimicrobial, etc. agents.
Keywords: 1,2,4 triazole, Utilization of Triazole moiety, Antineoplastic.
INTRODUCTION
Bladin first used the term “triazole” in 1885 to refer to a heterocyclic aromatic ring structure with five members and three nitrogen atoms with the chemical formula C2H3N31. White to pale yellow in color, it is a crystalline solid. It has a mild, distinctive scent and is accessible in both alcohol and water. Triazole derivative SAR studies reveal that substitutions at the triazole ring’s positions 3, 4, and 5 can be changed, but the cluster connected to the nitrogen molecule at position 4 has the biggest impact on physicochemical properties and biological profiles2.
The most difficult and gratifying area of chemistry is heterocyclic chemistry, and heterocycles are by far the most prevalent class in organic chemistry. In industrial applications, the vast majority of medicines, physiologically active agrochemicals, additives, and modifiers are by definition heterocyclic3. For the benefit of humanity, synthetic organic chemists achieved tremendous advancements in the discovery and development of a wide variety of heterocyclic molecules. Heterocycles’ extraordinary capacity to accept the substituent in the region of a middle structure is one of their structural qualities that are still used. The chemistry of nitrogen and sulfur-possessing heterocyclic compounds has advanced significantly over the century since their initial usage in agriculture4.
For this review article, we have screened more than 50 articles. Figure 1 shows the flow of selecting the literature to be reviewed.
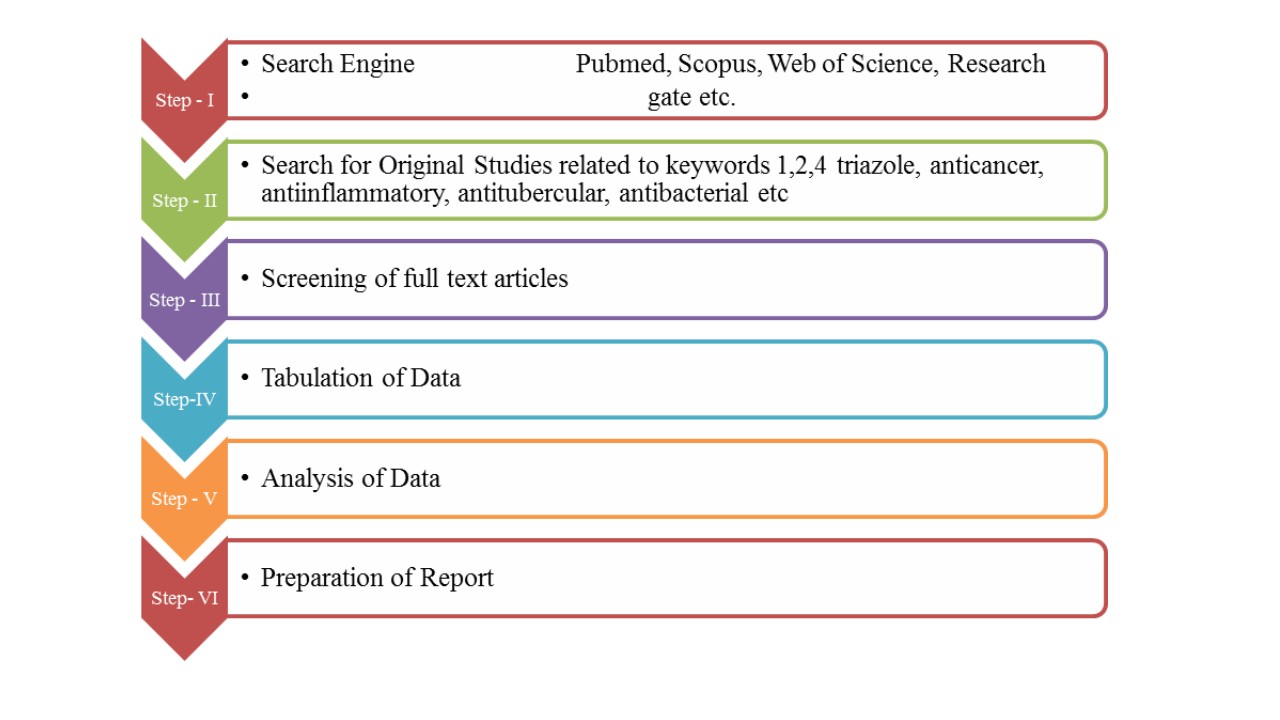
Figure 1: Shows the flow of selecting the literature to be reviewed.
Triazoles are potential bioactive agents due to their wide spectrum of therapeutic importance. Drug molecules having 1,2,4-triazole nucleus with good activity are listed under,
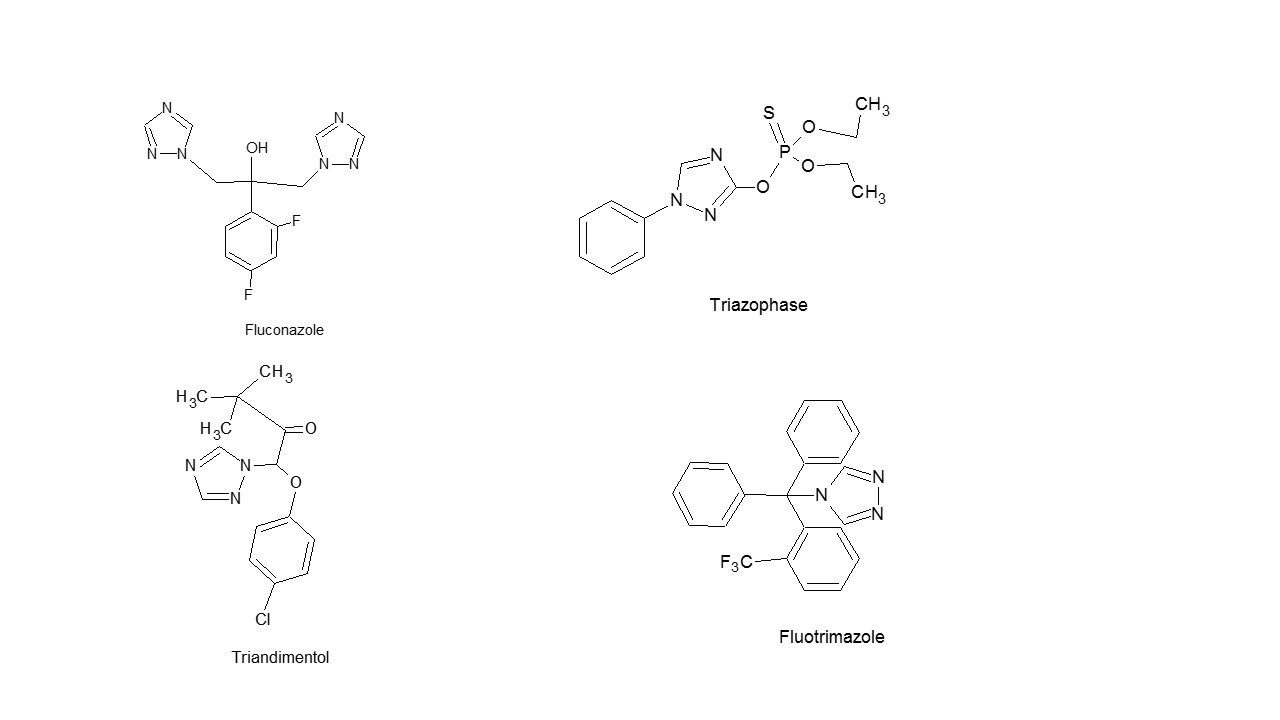
- Chemistry:
There are two possible isomers of triazole (R1, S1) depending on the position of nitrogen atom in the ring and are numbered as shown below. (Fig. 2) 5.
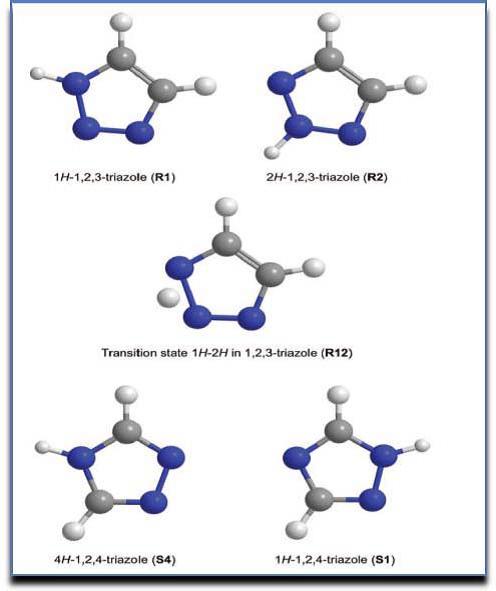
Figure 2: (R-1, R-2, S-1 and S-4) Triazole tautomers and alteration position (R12).
- Skeletal Features of Triazoles
2.1. Aromaticity and Stability
Aromatization is primarily responsible for the triazole nucleus’ stability. Nitrogen atom donate two electrons, one electron from each molecule attached by a double bond, resulting in the formation of an aromatic sextet6. Triazole nuclei can also be represented by tautomeric forms since resonance stabilizes them7.
2.2. Triazoles exhibit tautomerism
In two possible isomers of triazole tautomerism is possible.
2.2.1:1,2,3-Triazole Tautomerism:
Mentioned molecules come in two tautomeric forms: The triazoles 1H-1,2,3 and 2H-1,2,3 are two examples. (2)8.
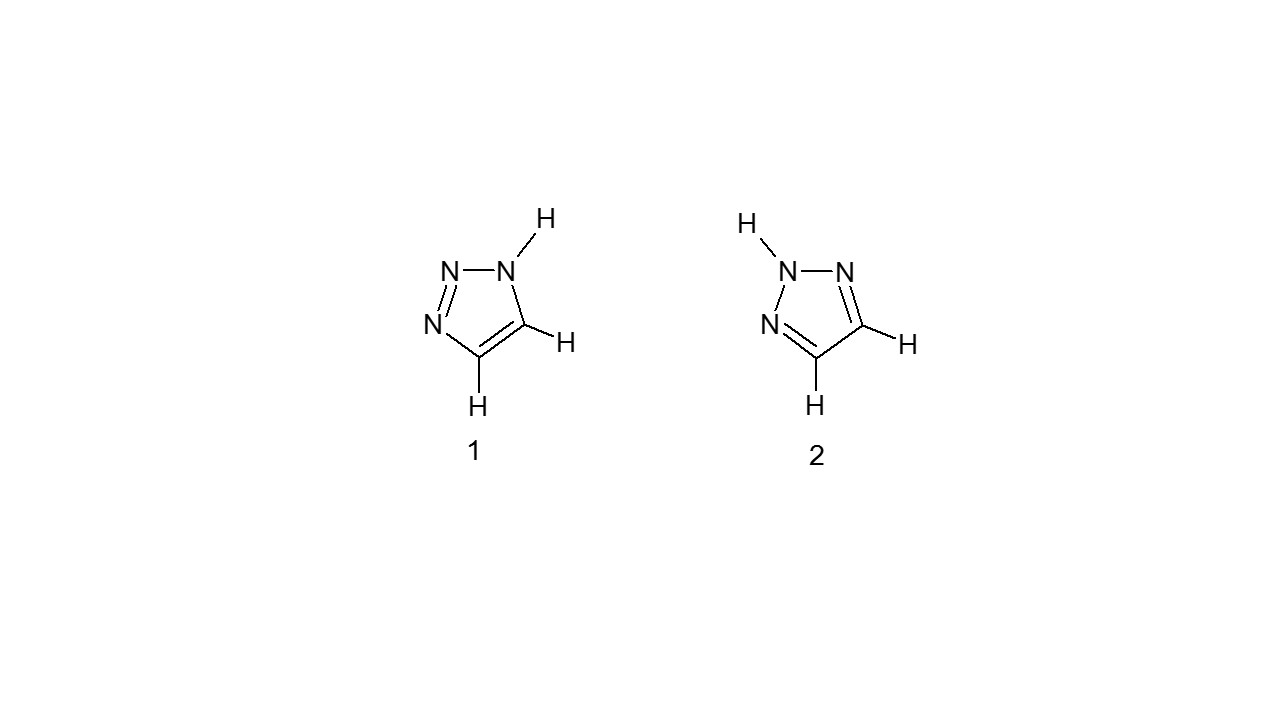
2.2.2 :1,2,4-triazoles Tautomerism
Additionally, 4th position Hydrogen containing triazole and 1st position Hydrogen containing triazole are the mentioned tautomeric form of 1, 2, 4-triazoles (3). The compound (3) is highly steady than the compound (4), according to a number of studies9.
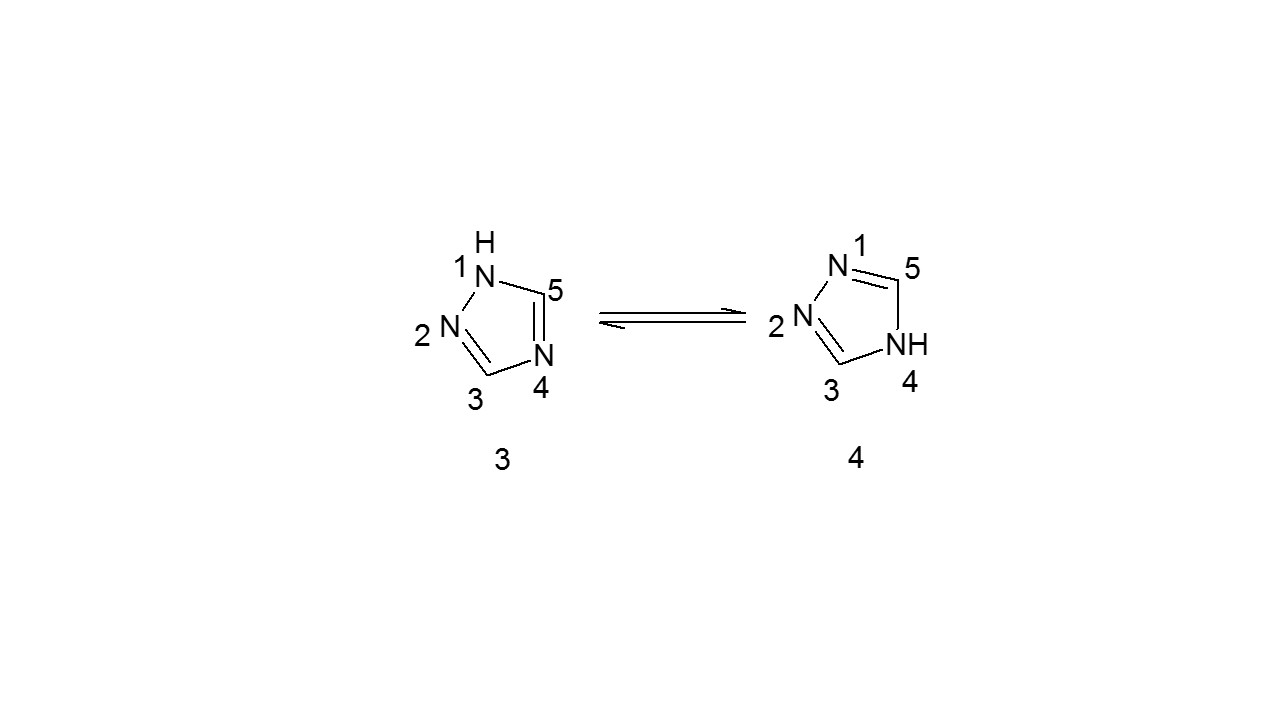
2.2.3: Tautomerism arises in 1,2,4-triazoles that have been substituted:
One of the substituted 1,2,4-triazoles is the 3-SH-1,2, 4-triazole, which has two tautomeric forms that allow mobile hydrogen to link to either nitrogen (thion form) (5) or sulphur (thiol form) (6)10.
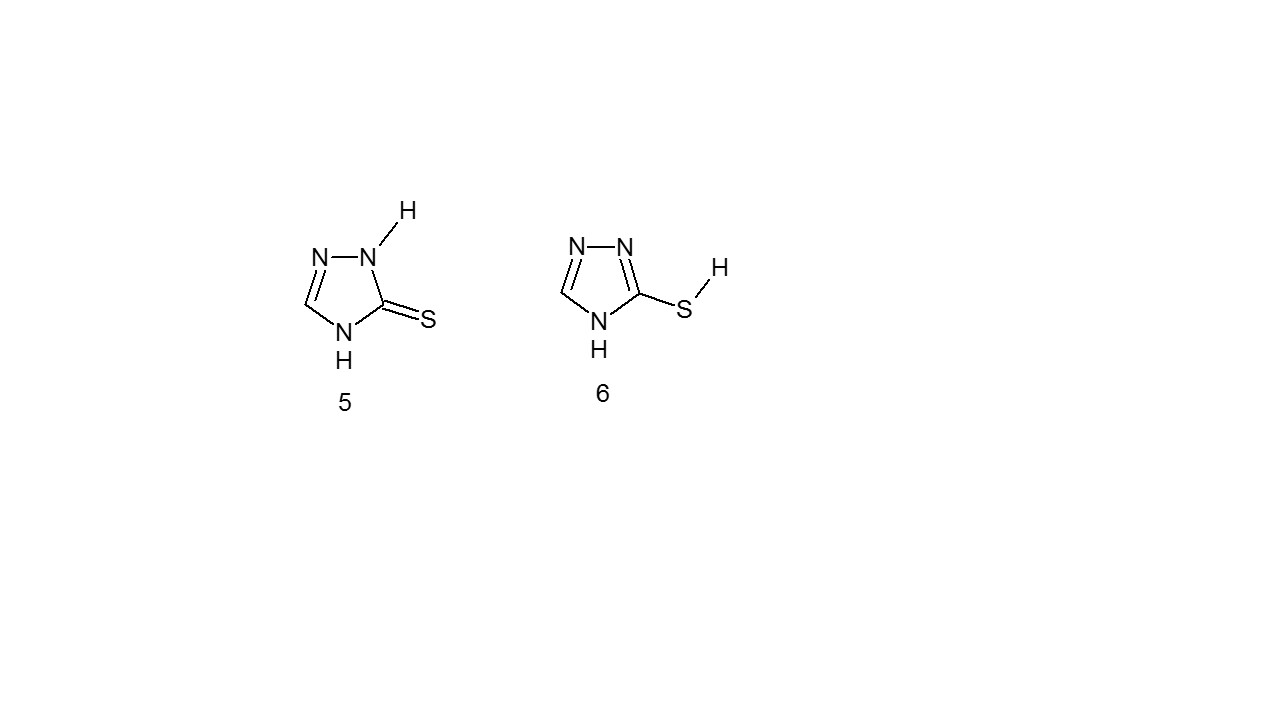
- Spectroscopy
3.1 Ultraviolet spectroscopy (UV)
The mentioned compound has a very fine absorption at 205 nm in the (UV) absorption peak, according to Holam and Straub. On the other hand, N-Acetyl-1,2,4-triazole (7) displays a redshift with a peak at 221.5 nm. 3,5-dimethyl-1,2,4-triazole (8) has a comparable change in its maximum absorption to the compound (9)11.
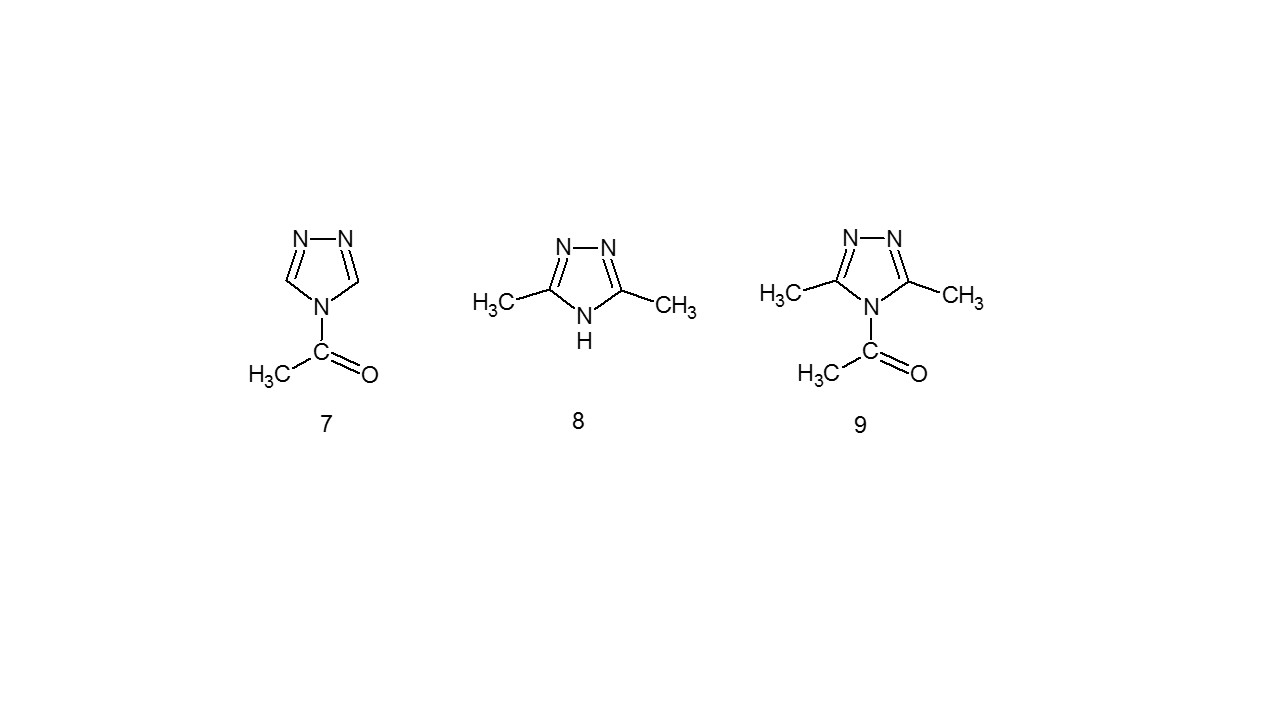
3.2 Infrared Spectroscopy (IR)12
A key factor in classifying triazole compounds is infrared spectroscopy. The diagnostic features are the % transmission spectra at 1572-1548 cm-1 reflect N=N functions and nearby 1601-1400 cm-1 induced by C=N functions.
The absorption band at about 2710-2560 cm– 1 for thiol forms, the C=S absorption peak found at 1258-1166 cm-1 in support of thion group are found in the thion-thiol tautomeric forms of mercapto-1,2,4-triazoles.
Similar evidence has been found for two weaker spectrum at 3350 cm-1 and 3250 cm-1 supporting thion-thiol equilibrium have been associated with the fundamental N-H stretching vibrations. N-H bands can also be seen in the 3200–3100 cm-1 area.
- Pharmacological Applications
The biological and pharmacological characteristics of 1,2,4-triazoles have received a great deal of interest in recent years in terms of their synthesis and characterisation13. The pharmacological actions of triazole moieties quite diverse Figure (3).
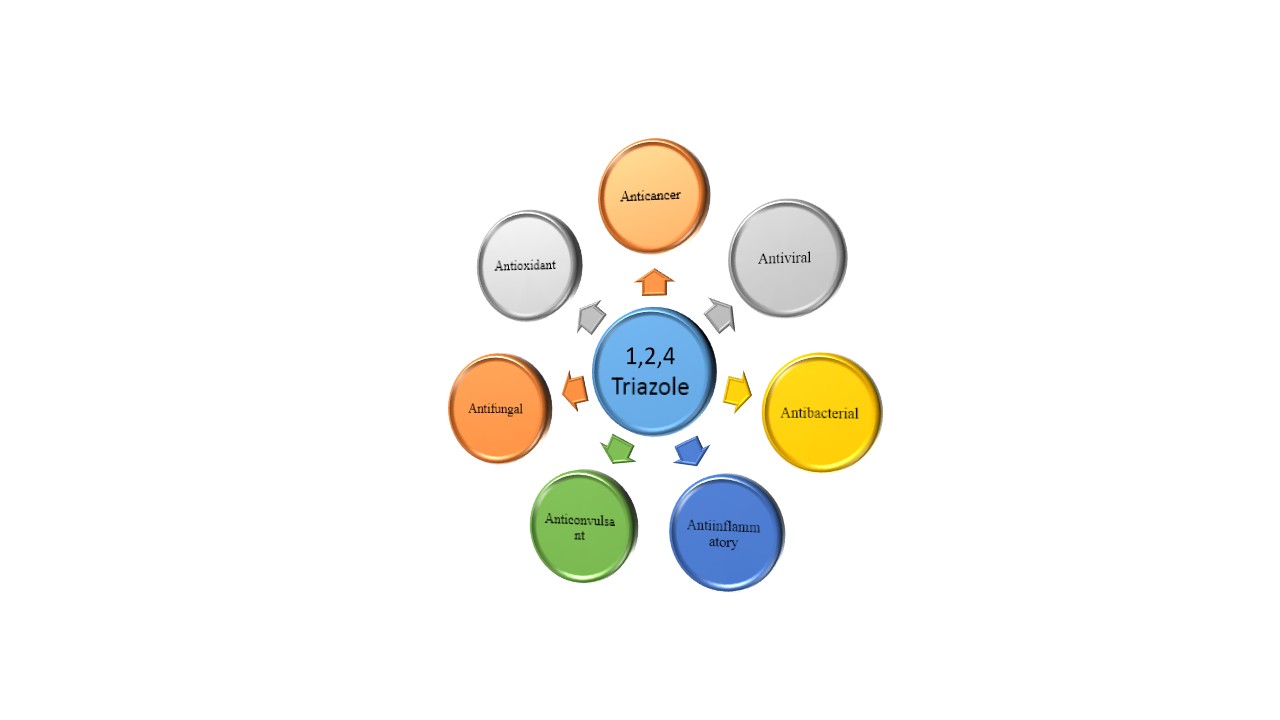
Figure 3: Triazole Moiety showing different pharmacological activities.
4.1. Antibacterial Activities
A vast range of antibiotics have been developed in the fight against bacterial infections. Bacteria are developing antibiotic resistance as a result of years of abuse and overuse of antibiotics, potentially posing a threat to global health. Utilizing new antibacterial products with improved broad spectrum potency is advised. Therefore, research into new antibacterial drugs has been focused recently. Antibacterial drugs are chemicals derived from bacteria or mould be able to destroy germs to treat bacterial infections. Today, numerous antibiotics are structurally altered versions of naturally occurring substances.
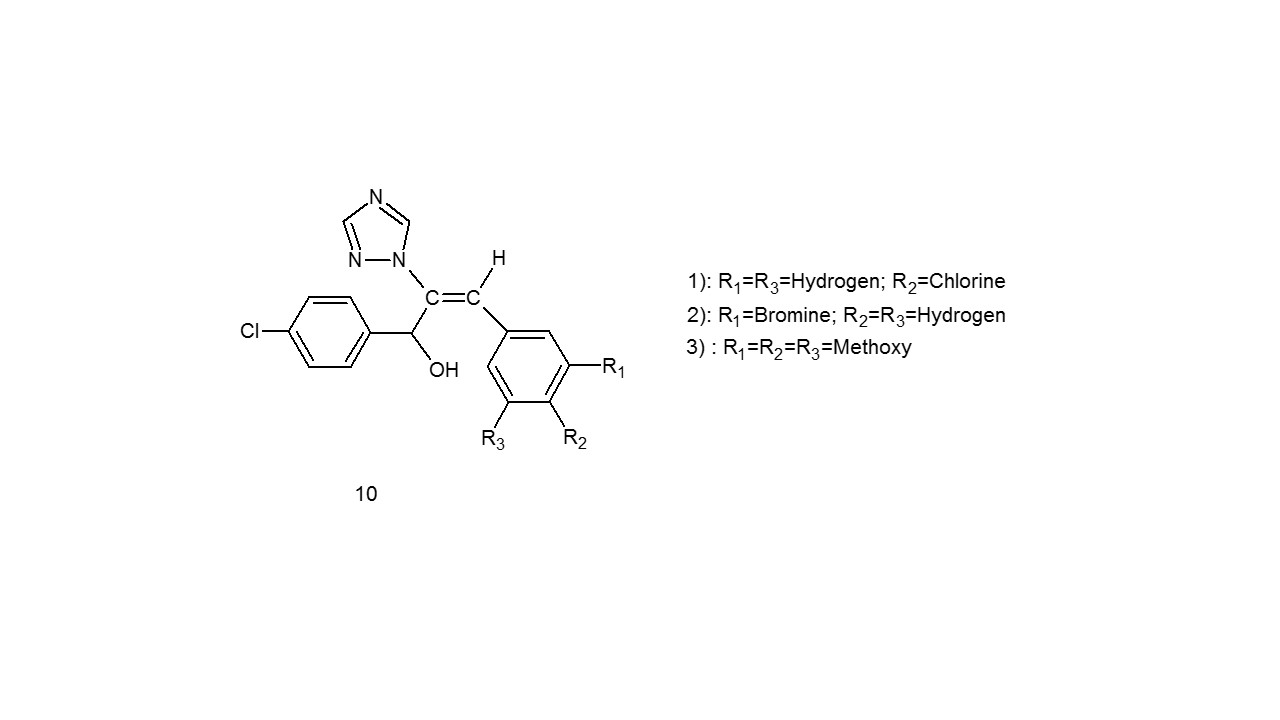
Based according to how they work, they can be divided into two groups: bactericidal agents (which directly kill bacteria) and bacteriostatic agents (which prevent germs from multiplying)14. Uchil et al.15 were produced and employed as bacteriostatic agents in substituted compound (10).
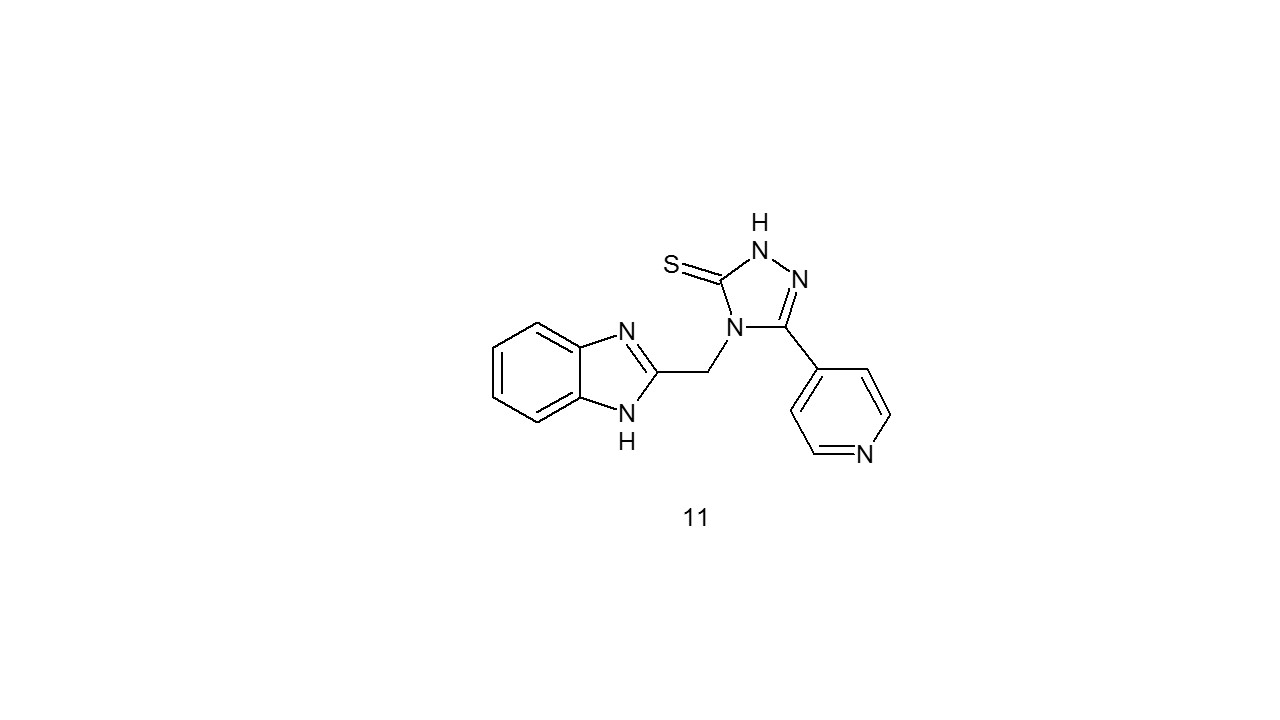
In order to assess how many unique 1,2,4-triazole-5-thione benzimidazole substituents might exhibit antimicrobial properties, Barot et al. synthesised a large number of them (11). Few of the derivatives shown effective antimicrobial properties. The stains had action against B. cereus, E. faecalis, S. aureus, Escherichia coli, P. aeruginosa, and K. pneumonia as well as antifungal activity against C. albicans, A. niger, and F. oxyspora. For antifungal activity, fluconazole was used, while metronidazole and ofloxacin served as benchmarks for antibacterial activity16.
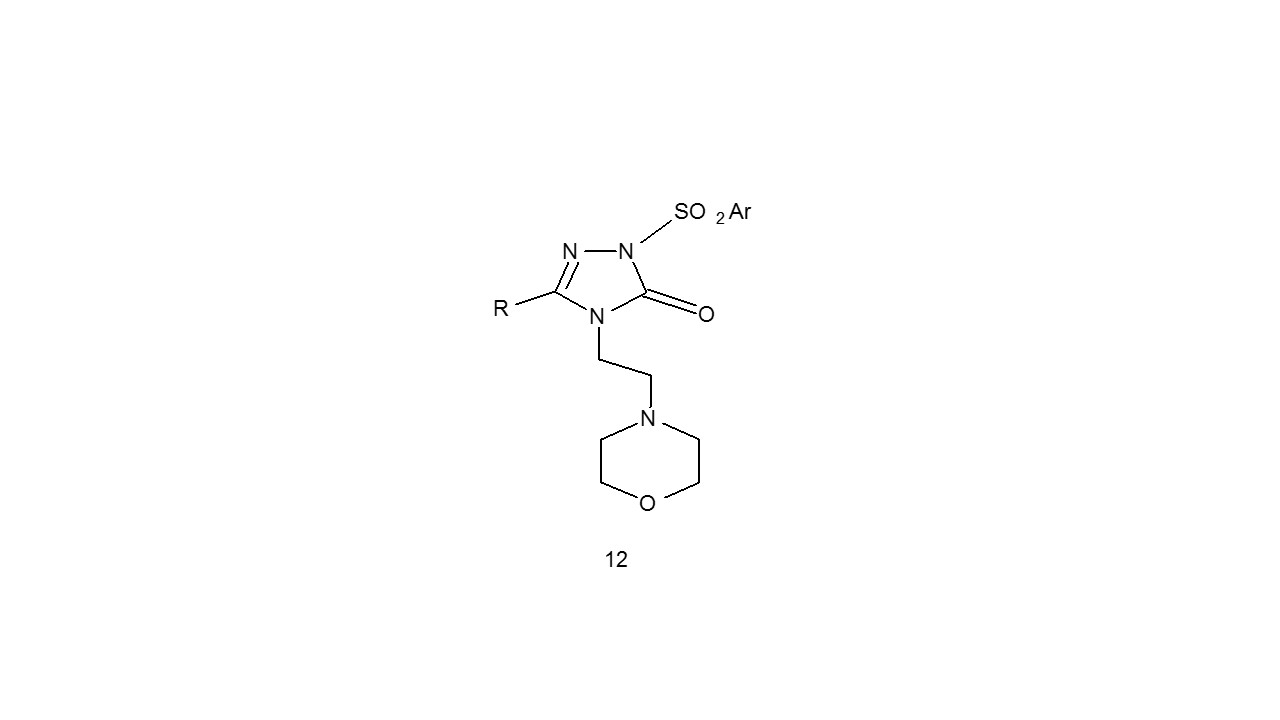
Novel triazole compounds with morpholine moiety (12) were synthesised by Sahin et al., and their antibacterial efficacy was tested. A. niger, C. albicans, C. tropicalis, E. coli, E. aerogenes, Pseudomonas aeruginosa, Staphyloccocus aureus, Enterococcus faecalis, Baccilus cereus were some used as test organisms.
The typical medications were Fluconazole, Streptomycin, and Ampicillin. The majority of the substances showed excellent antibacterial activity that was on par with that of conventional medications17.
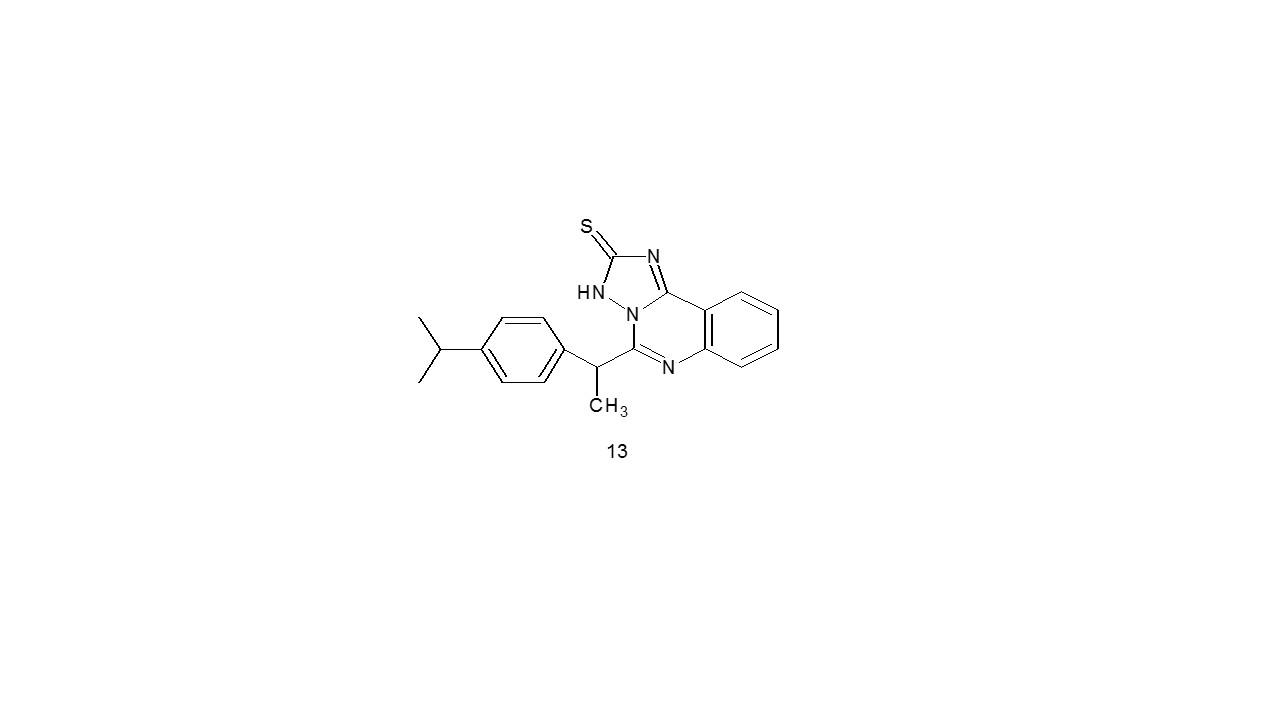
Abdulla et al. produced very good yields of ibuprofen derivatives (13) by cyclization under varied reaction conditions. Using the cup-plate method, the novel compounds’ microbial inhibitory action has evaluated in-vitro in opposition to S. aureus (gramme +ve) and E. coli (gramme -ve). In comparison to other compounds and conventional medications, three compounds shown the highest antibacterial activities18.
4.2. Antiproliferative Activities
Cancer, a broad category of disorder characterized by the emergence and growth of malformed cells, is a significant problem on a global scale19. Therefore, new effective and selective anticancer medication development and discovery are crucial to current cancer research. With these studies and their positive outcomes, 1,2,4-triazole compounds have a chance.
Lee et al. Twelve hybrid 1,2,4-triazole Schiff bases (14) with butenolide moieties were generated and tested for in vitro anticancer efficacy among them20.
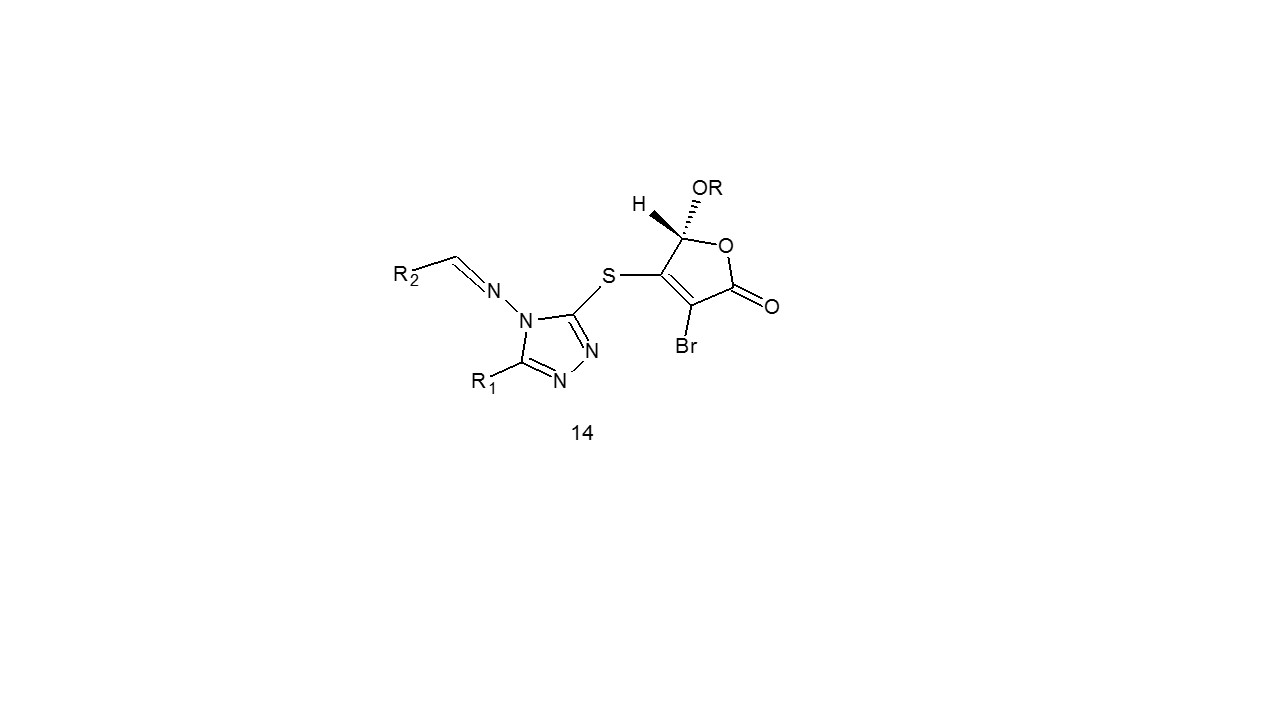
a: R=1-menthyl; R1=C6H5; R2=4-Cl-C6H5
b: R=1-menthyl; R1=4-CH3O-C6H5; R2=4-Cl-C6H5
c: R=1-menthyl; R1=C6H5; R2=4-O2N-C6H5
d: R=1-menthyl; R1=4-CH3O-C6H5; R2=4-O2N-C6H5
e: R=1-menthyl; R1=C6H5; R2=2-furanl
f: R=1-menthyl; R1=4-CH3O-C6H5; R2=2-furanl
g: R=1-menthyl; R1=CH3; R2=4-Cl-C6H5
h:R=1-menthyl; R1=4-HO-C6H5; R2=4-CH3O-C6H5
i:R=1-menthyl; R1=C6H5; R2=4-HO-C6H5
j: R=1-bornyl; R1=C6H5; R2=4-Cl-C6H5
k:R=1-bornyl; R1=4-HO-C6H5; R2=4-CH3O-C6H5
l:R=1-bornyl; R1=CH3; R2=2-HO-C6H5
Three different (substituted phenyl) 1,2,4-triazolo- 1,3,4-thiadiazines (15) were created by Bhat et al through the cyclization of 1,2,4-triazol-5-thiol with substituted phenbromides and then their anticancer potency was examined. A panel of 60 tumour cell lines including breast, prostate, ovarian, lung, leukaemia, melanoma, out of sixty compounds, 3 of them showed cytotoxic potency with moderate to good growth suppression. With GI50 values between 1.06-25.4, one substance demonstrated positive antiproliferative activity21.
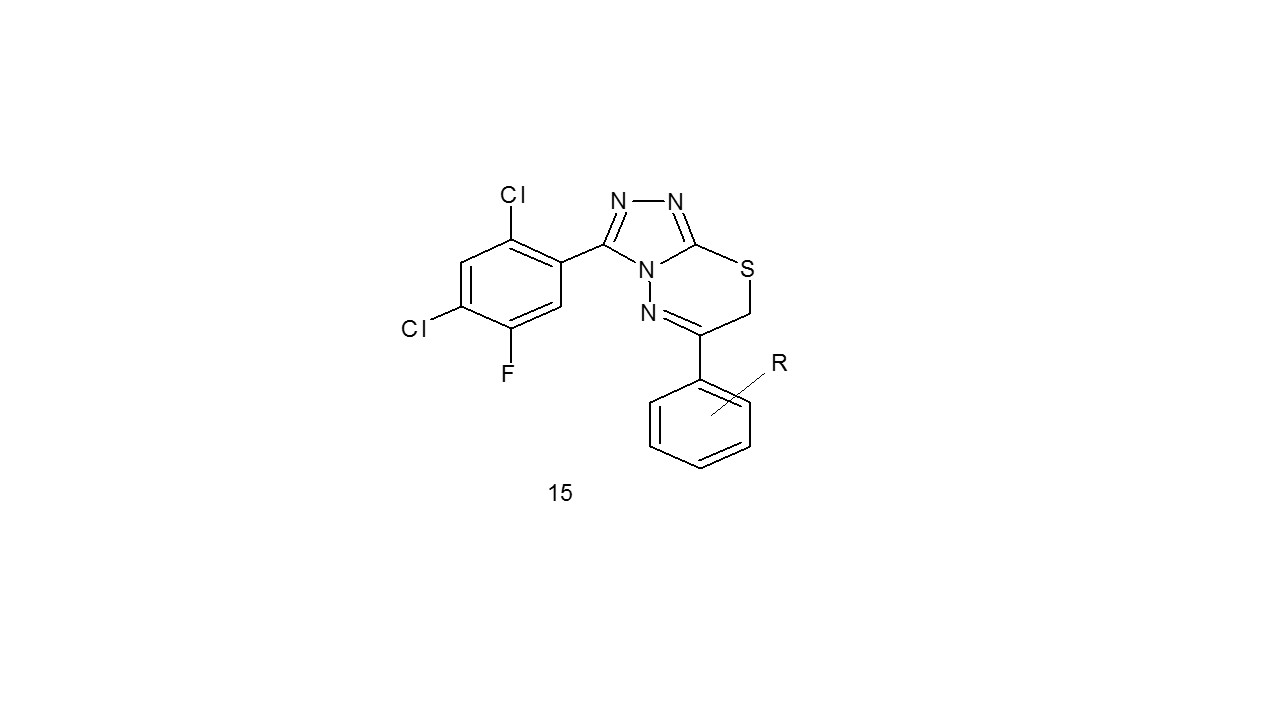
R- Hydrogen; 4-Chloro; 4-Bromo; 4-Methyl; 4-Methoxy
R- C6H5; 4- methoxy phenyl; 4-Bromo phenyl
2-[4-Substituted-phenyl-1,2,4-triazol] was created by Fattah et al. Using the 4-tosylamino benzohydrazide as the starting point, N-substituted acetamides (16) their ability to kill the breast maligancy cell line (MCF-7) and the colon cancer cell line (HCT-116). Two compounds showed noticeable action in opposition to the mentioned cell lines in the range of 3.1-11.1 g/ml, according to the results of the cytotoxic activity. The most popular medication prescribed was doxorubicin22.
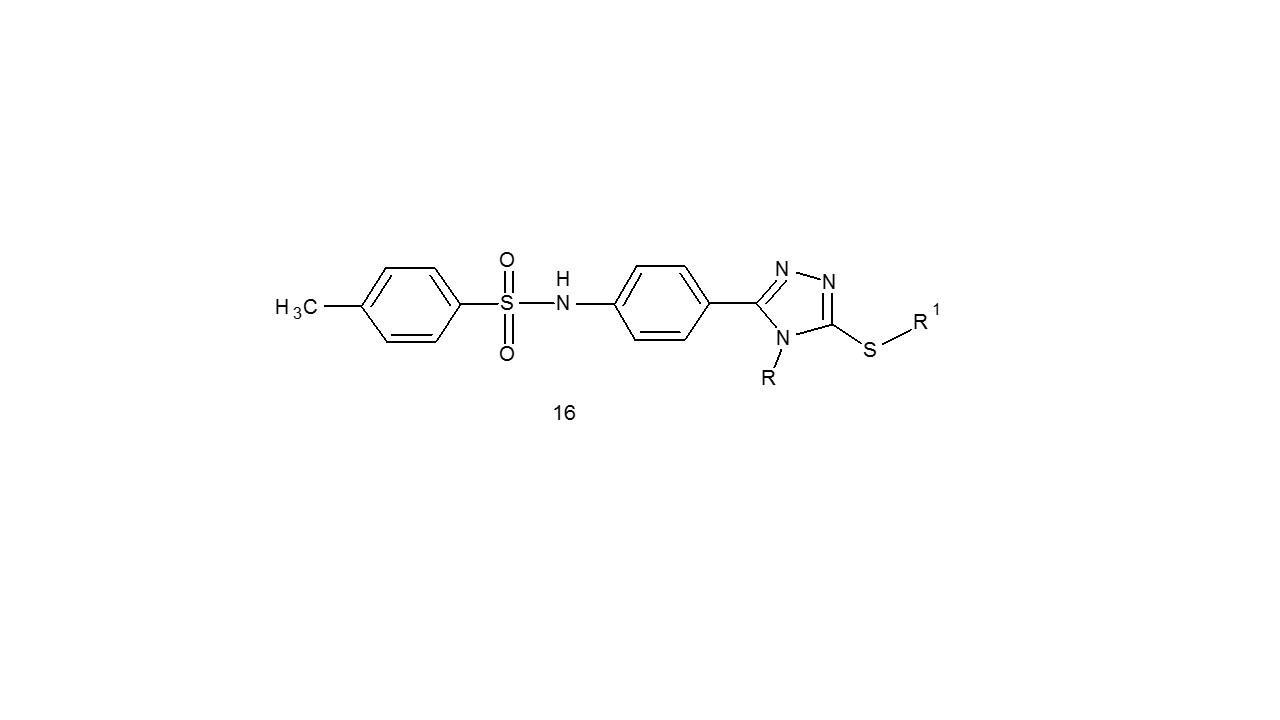
R- Phenyl; 4-methoxyphenyl; 4-bromophenyl.
R1- ethyl;
Li et al. reported the twelve hybrid 1,2,4-triazole Schiff bases bearing γ-substituted butenolide moiety. These compounds were synthesized by utilizing the tandem asymmetric Michael addition/elimination reaction as the key step. (17). In-vitro anticancer potential of the entity against HeLa was evaluated using MTT test.
All the medications shown effective inhibitory potential on cervical cell lines. The substance had the highest level of growth suppression action, with an IC 50 value 1.8M23.
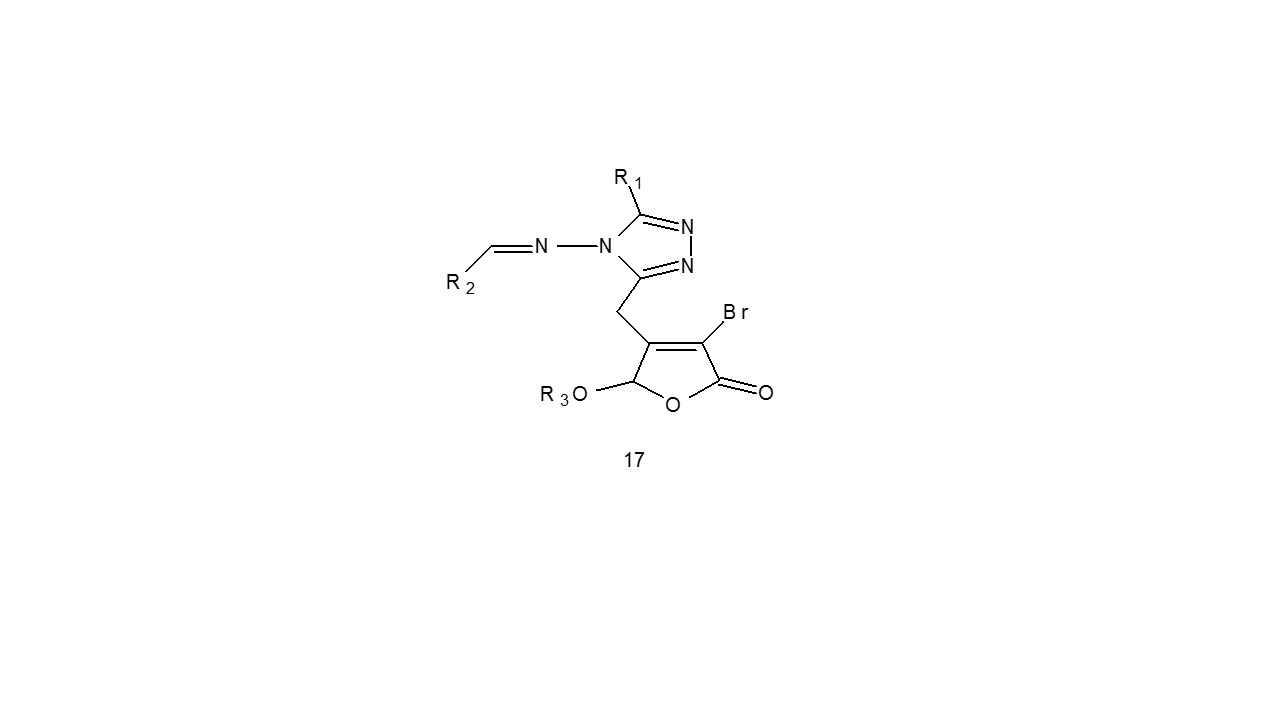
R1- Methyl; Ethyl R2- 4-nitrophenyl; 4-hydroxyphenyl, R3- 1-menthyl; bornyl
4.3 AntiTubercular
The anti-tubercular activity of isopropylthiazole merged triazoles (18) was tested utilising the broth dilution assay method by Kumar et al. against the Mycobacterium tuberculosis H37Rv strain. The outcomes demonstrated that some of the compounds demonstrated interesting actions at Minimum Inhibitory Concentration 16–16.5 Kg/L, and are deserving of additional study24.
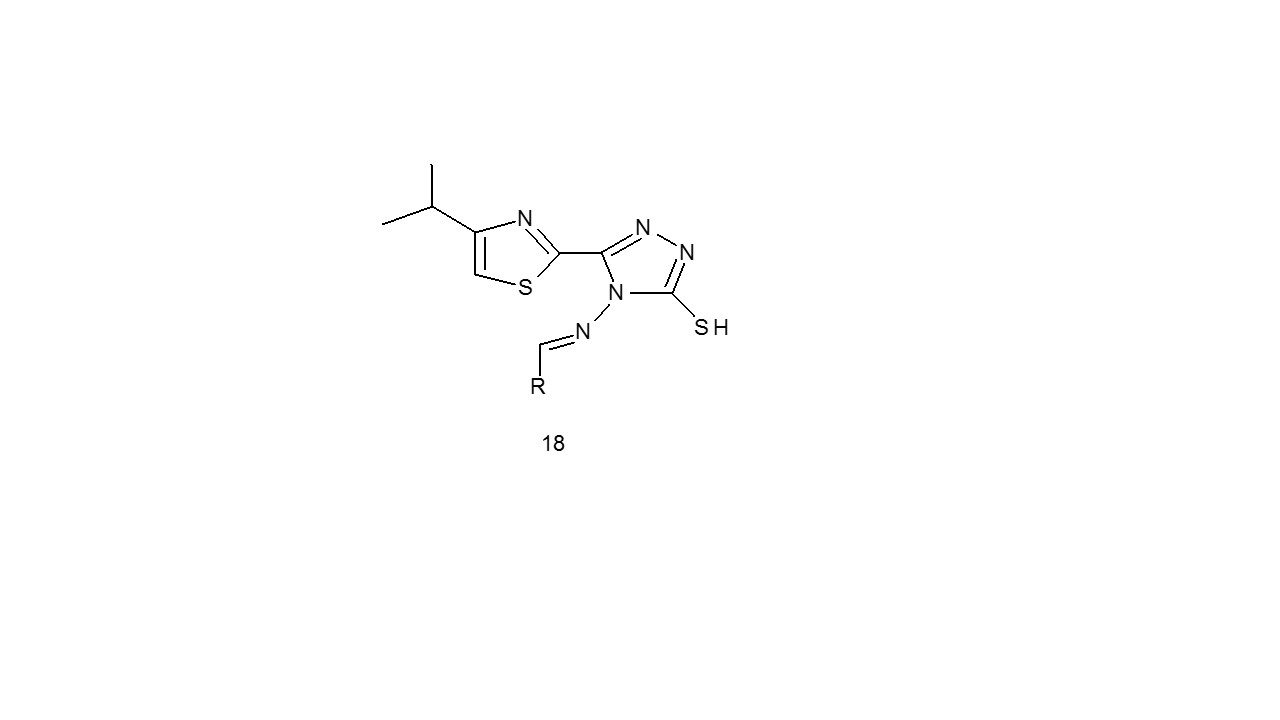
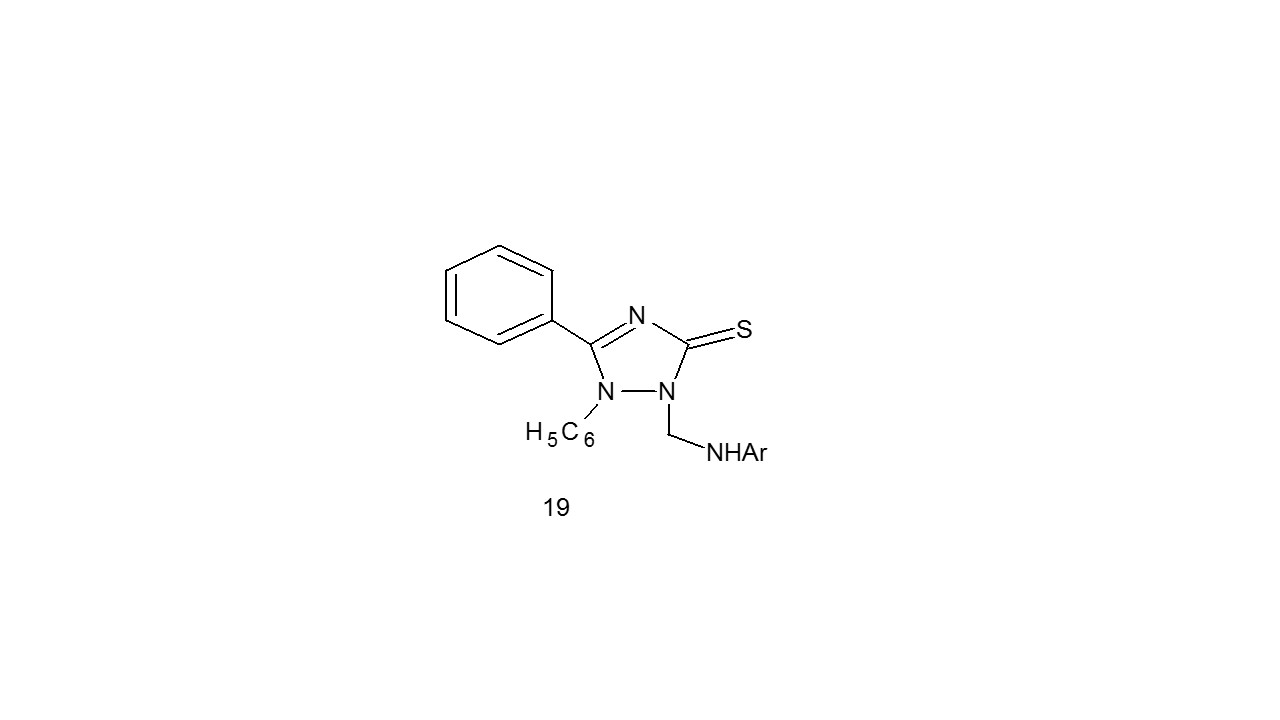
Godhani et al. developed a series of compounds (19) and tested using the LJ slope technique for antitubercularl activity against the M. tuberculosis (H37Rv). In comparison to the standard medicine Isoniazid, four compounds had good anti-tubercular action at 250 g/ml. The electronegative character of substituent groups determines the activity25.
4.4 Anticonvulsant activity
In a two-step synthesis of compound (20) and checked them for anticonvulsant activity using the MES test. Because of failure to pass the BBB, compounds containing lengthy alkyl chains at the 4 position of the 1,2,4-triazole moiety and no alkyl substituent at the N-3 position lacked the protective anticonvulsant action. When compared to the conventional drug Valproate, having an alkyl fragment at position 4 of triazole nucleus demonstrated high affinity and potency in mice at concentration of 300mg/Kg26.
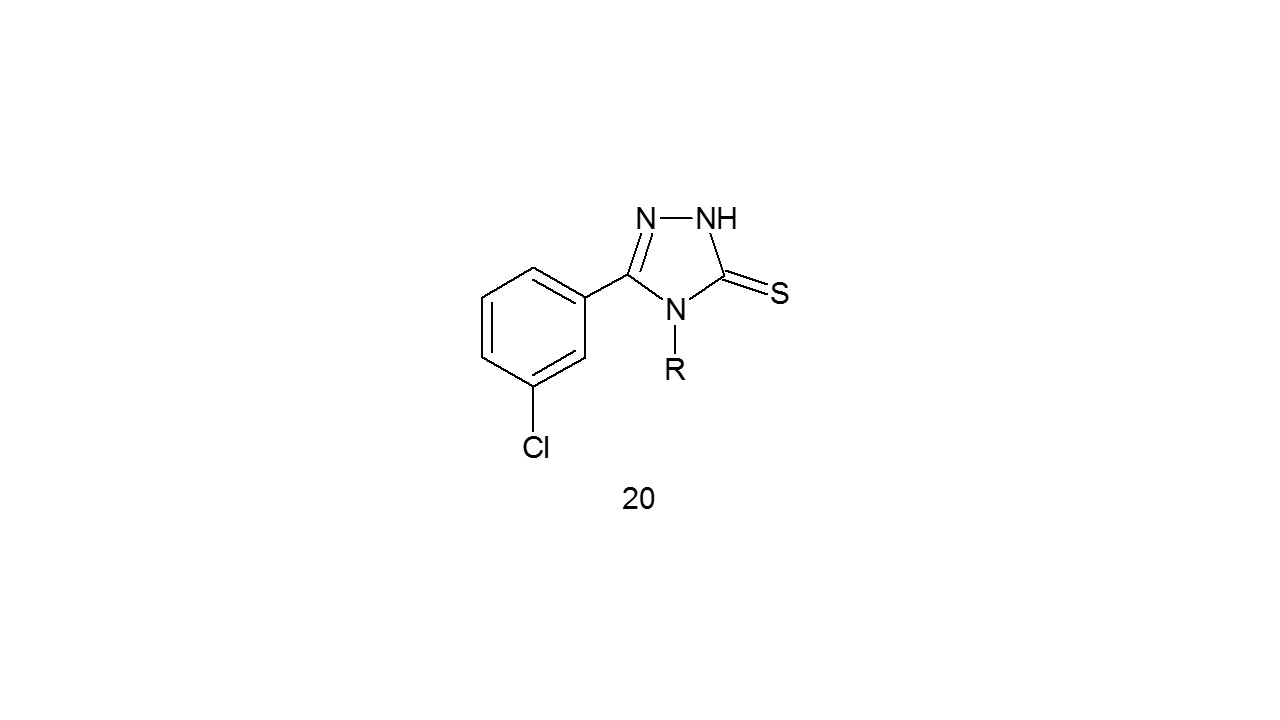
R- C2H5; C4H9; C5H11; C6H13; C7H15; C9H19
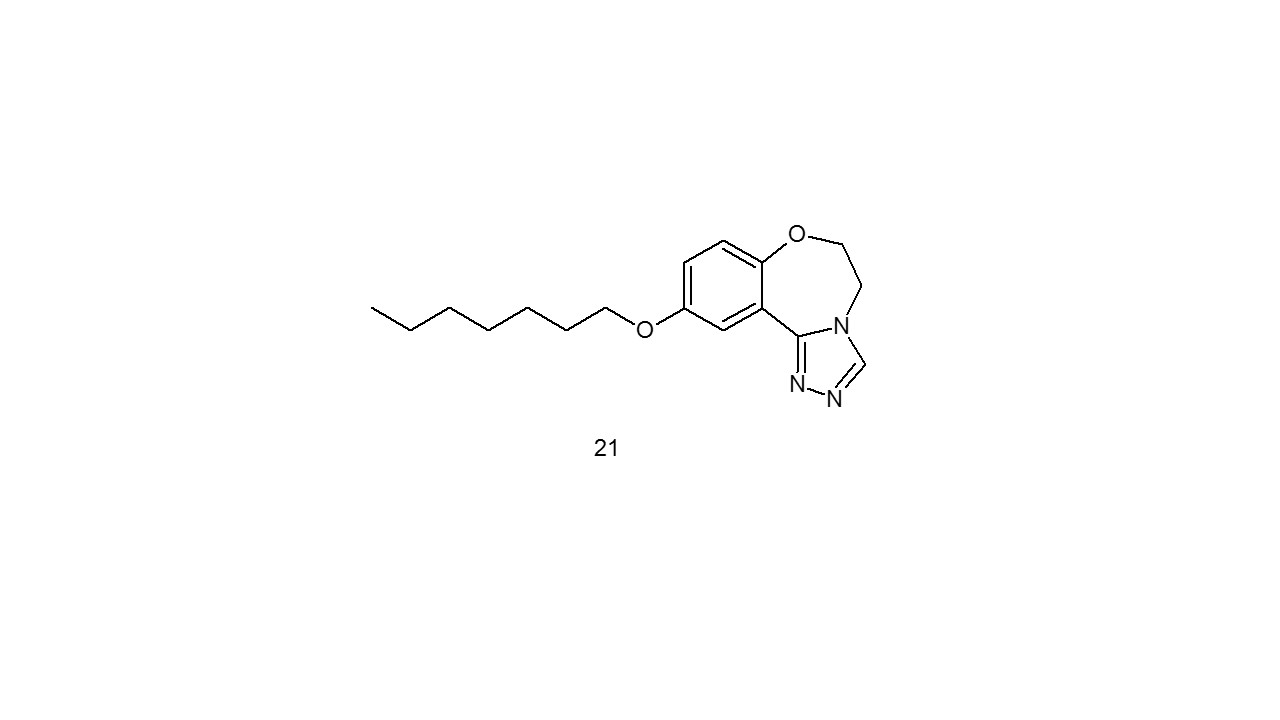
In contrast to commercially available medications carbamazepine and phenytoin compound 10- (21) demonstrated superior anticonvulsant action and highly safe (ED50= 6.9 mg/Kg). In comparison to carbamazepine and phenytoin, which have Protective Index values of 6.4 and 6.9, respectively, the neurotoxicity value of 65.7 µg/gm was detected, yielding a high protecting Index value of 9.527.
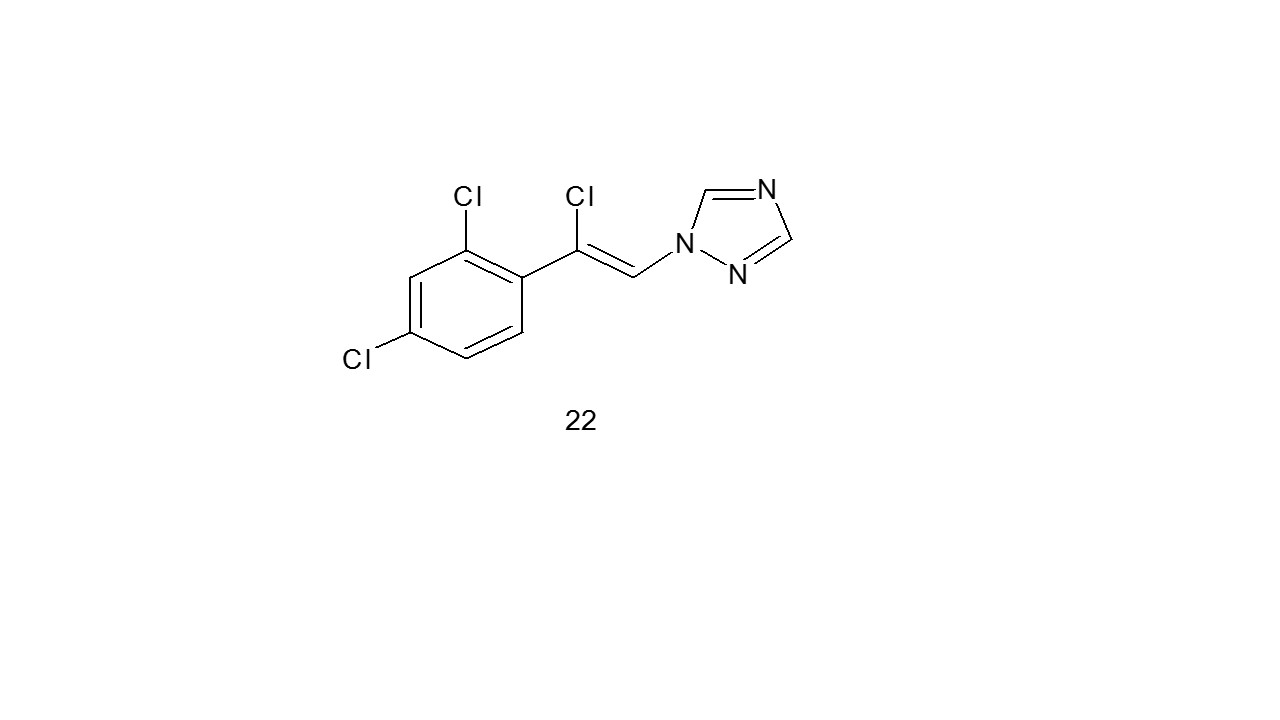
Wingrove et al. proposed the theory that the affection in the triazole nucleus and the amide Asn-289, which is situated on the â2 subunit of the Gamma Amino Butyric Acid A receptor, is what makes loreclezole (22) (a second-generation antiepileptic medication) active28.
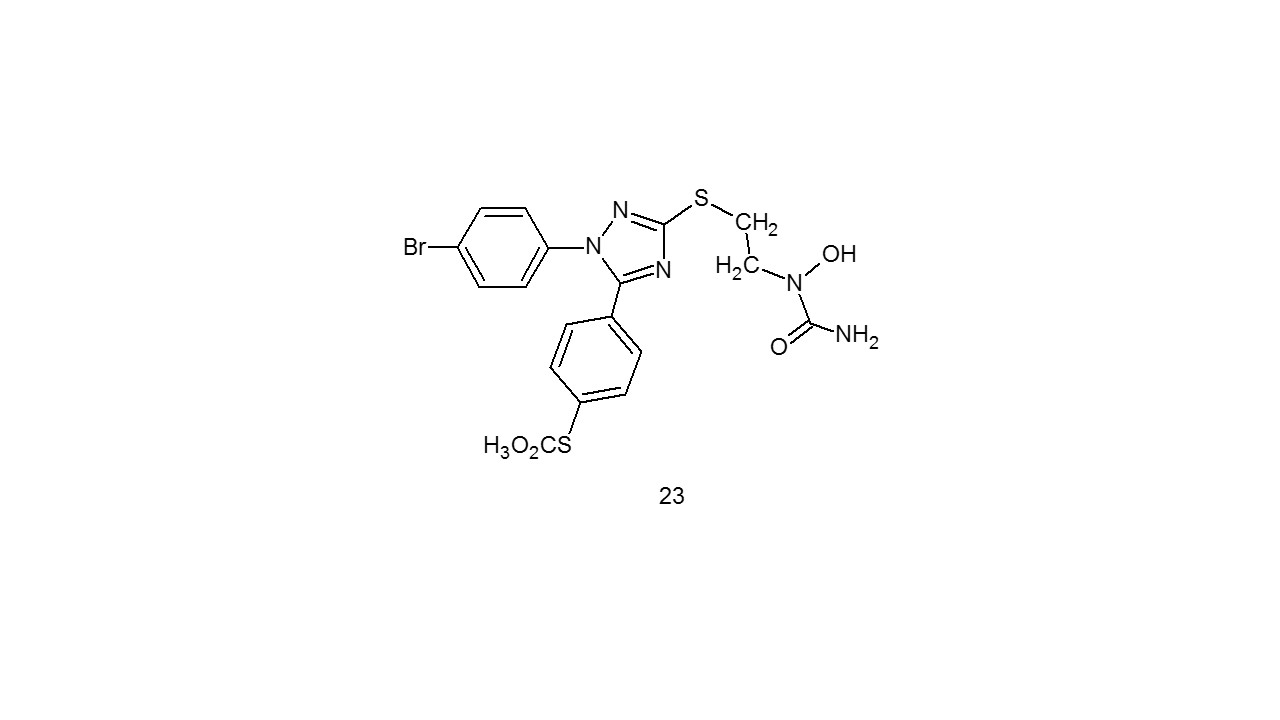
4.5 Anti-inflammatory Activities:According to Jiang et al.29 Numerous hybrids formed from triazole and urea (23) were developed, evaluated as potent anti-inflammatory medicines, and demonstrated encouraging analgesic potential in hot-plate experiment and acetic acid-induced writhing response.
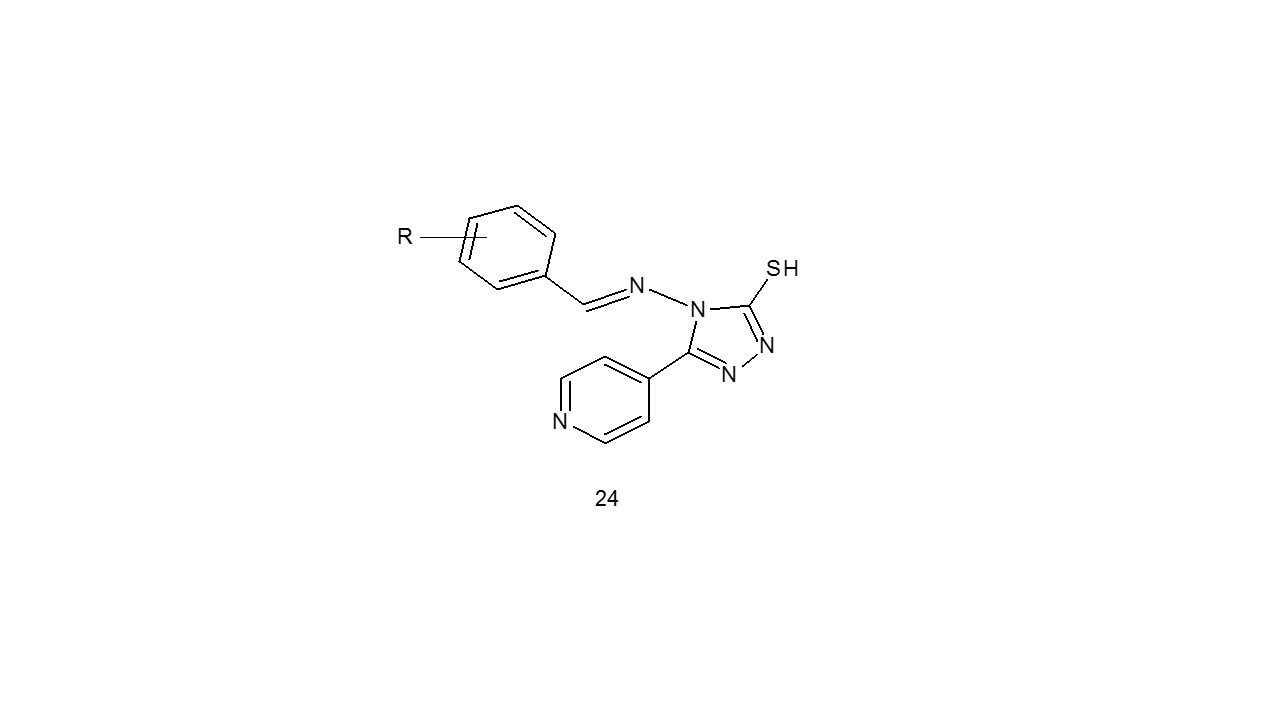
The anti-inflammatory activity of compound (24) have been studied by Murti and coworkers30.
4.6 Antiviral activity
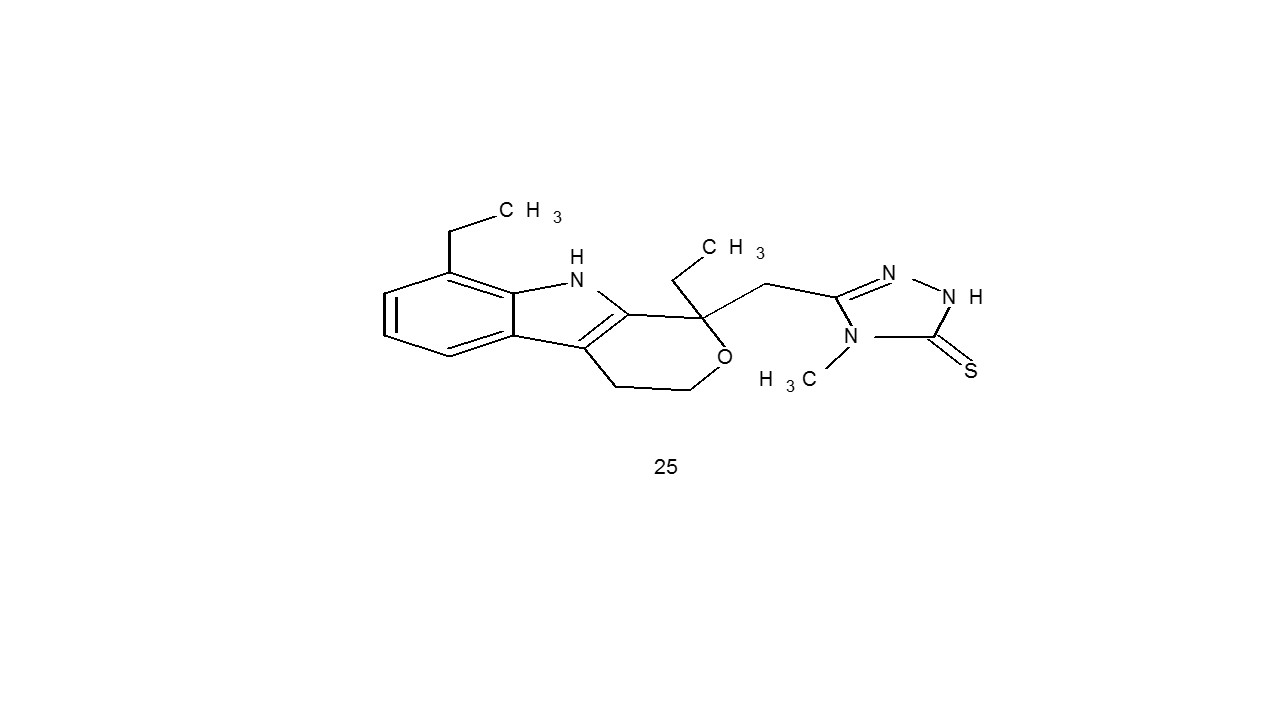
Suzgun and coworkers created compound of etodolac (25) and tested them for antiviral efficacy against HCV NS5B polymerase. The most active chemical was identified as 4a, having an IC50 value of 14.8 M. The compound used here for comparison, etodolac, produced a 10% inhibition of NS5B polymerase, whereas its substituents showed a 5.0–80.0% suppression of NS5B polymerase activity31.
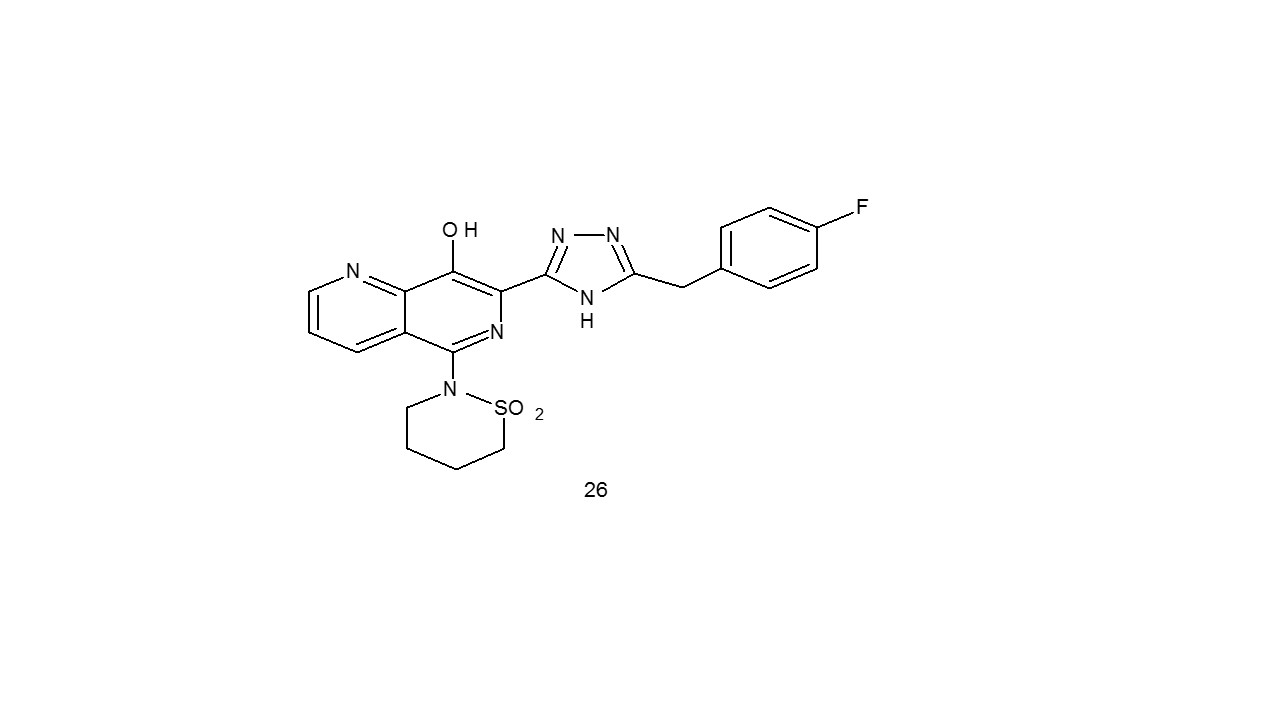
A group of Integrase inhibitors with unique metal binding motifs made of the 8-OH-1,6-naphthyridine moiety and triazole were created by Johns et al (26). The antiviral activity of the produced drugs was tested. Early analysis of the C5 alteration revealed appreciable increases in antiviral activity32.
Applications in Industry
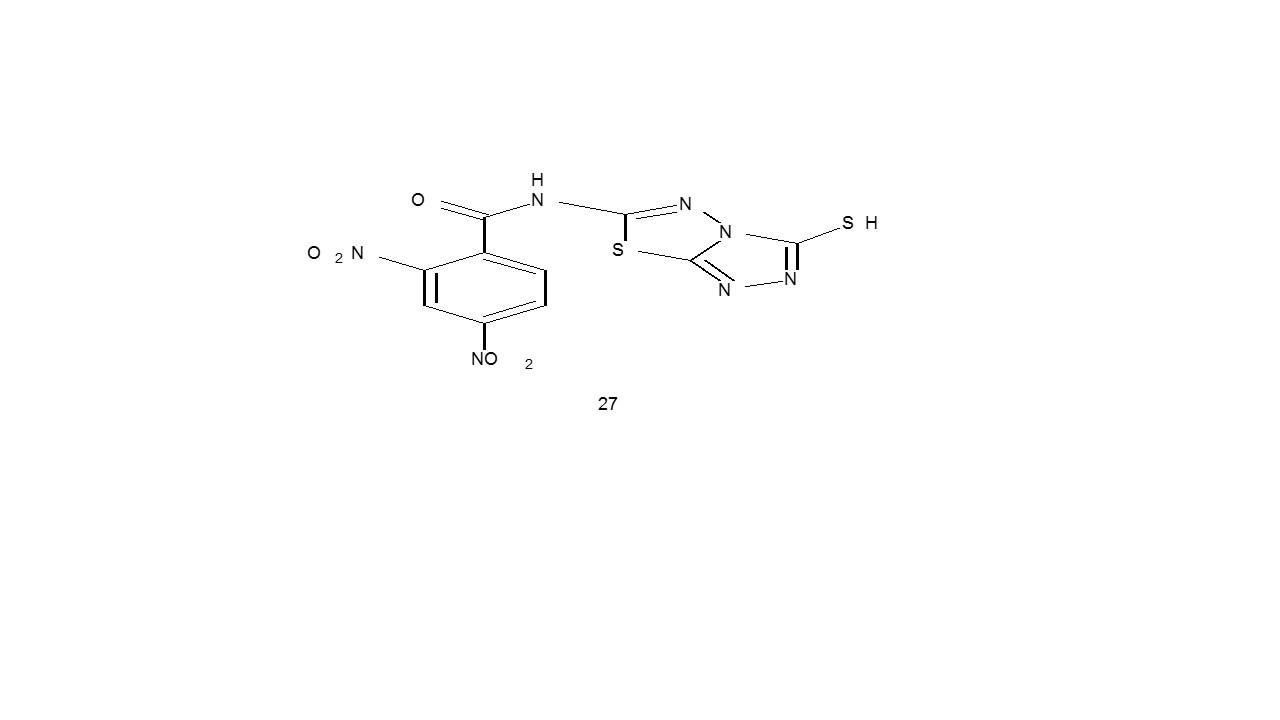
Choosed triazoles have been employed as electroluminescent devices33. Numerous triazole types, such as 2-mercapto-1,2,4-triazole (27) utilized to boost, the effectiveness of cooling fluids 34.
REFERENCES:
- Bladin, J. A. Ueber von dicyanphenyl hydrazin abgeleitete verbindungen. Ber. Dtsch. Chem. Ges. 1885; 18:1544–1551. Doi:10.1002/cber.188501801335.
- Asif M. A brief review on antitubercular activity of pharmacological active some triazole analogues. Glob J Res Rev. 2014; 1(3):51–58.
- Katritzky A.R, Ress C.W, Scriven E. Comprehensive heterocyclic chemistry II., Eds. Pergamon: Oxford, U.K., 1996, 1-9.
- Katritzky A.R, Ress C.W, Scriven E, Taylor R.J. Comprehensive heterocyclic chemistry III., Eds. Pergamon: Oxford,U.K., 2008, pp1-13.
- Balabin RM. Tautomeric Equilibrium and Hydrogen Shifts in Tetrazole and Triazoles: Focal-Point Analysis and ab-initio Limit. J. Chem. Phys. 2009; 131:1- 8.
- Koteleviskii SI, Prezhdo OV. Aromaticity Indices revisited: Refinement and Application to Certain Five- Membered Ring Heterocycles. Tetrahedron. 2001; 57:5715-5729,.
- Obot IB, Johnson AS. Ab initio, DFT and TD- DFT Electronic Absorption Spectra Investigations on 3,5-Diamino-1,2,4-triazole. Elixir Comp. Chem. 2012; 43:6658-6661.
- Ozimin´ski WP, Dobrowolski JCz, Mazurek AP. DFT Studies on Tautomerism of C5-Substituted 1,2,3- Triazoles. J. Mol. Str. 2003; 651-653, 697-704,.
- Chawla A, Kaur P. A Systematic “Review: Microwave Synthesis as A Part of Green Chemistry for the Synthesis of Novel 1,2,4-Triazole Derivatives. IRJP. 2013; 4(1):49-72.
- Pinto DC, Santos CM, Silva AM. Advanced NMR Techniques for Structural Characterization of Heterocyclic Structures. Recent Res. Dev. Heter. Chem. 2007; 37 (2):397-475.
- Holam SC, Straub BF. Synthesis of N-Substituted 1,2,4-Triazoles: A Review. New J. Org. Syn., 2011; 43 (4):319-347.
- Bahgat K, Fraihat S. Normal Coordinate Analysis, Molecular Structure, Vibrational, Electronic Spectra and NMR Investigation of 4-Amino-3-phenyl-1H-1,2,4- triazole-5(4H)-thione by Ab initio HF and DFT Method. Spectrochimica Acta Part A: Mol. Biomol. Spectro. 2015; 135:1145–1155.
- Rao DVN, Prasad ARG, Spoorthy YN, Rao DR, Ravindranath LK. Synthesis, Characterization and Pharmacological Studies of Sulphur Containing 1,2,4- Triazole Derivatives. J. Taibah Uni. Med. Sci. 2014; 9 (4):293-300.
- Singh G, Sharma P, Dadhwal S, Garg P, Sharma S, Mahajan N. Triazoles Impinging the Bioactivities. Int. J. Curr. Pharm. Res., 2011; 3 (2):105- 118.
- Uchil VR, Joshi V. Selective Reduction of Substituted α-(1,2,4-Triazol-1-yl)chalcones NaBH4 and Al-Isopropoxide: Synthesis of Substituted (+)α-(4- Chlorophenyl)-β-(phenylmethylene)-1H-1,2,4-triazole- 1-ethanols Having Potential Bacteriostatic and Agro- Based Fungicidal Activity. Indian J. Chem. 2002; 41B:631-634.
- Barot KP, Manna KS, Ghate MD. Design, synthesis and antimicrobial activities of some novel 1,3,4-thiadiazole, 1,2,4-triazole-5-thione and 1,3-thiazolan-4-one derivatives of benzimidazole. Journal of Saudi Chemical Society. 2013.
- Sahin D, Bayrak H, Demirbas A¸ Demirbas N¸ Karaoglu SA. Design and synthesis of new 1,2,4-triazole derivatives containing morpholine moiety as antimicrobial agents. Turkish Journal of Chemistry. 2012; 36:411-426.
- Abdulla IQ. Synthesis and antimicrobial activity of Ibuprofen Derivatives. Natural Science 2014; 6(2): 47-53.
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11, Lyon, France: International Agency for Research on Cancer. 2013.
- Li X, Li XQ, Liu HM,. Zhou XZ, Shao ZH. Synthesis and Evaluation of Antitumor Activities of Novel Chiral 1,2,4-Triazole Schiff Bases Bearing ã- Butenolide Moiety. Org. Med. Chem. Lett. 2012; 2 (26):1-5.
- Bhat KS, Poojary B, Prasad DJ, Naik P, Holla B. Synthesis and antitumor activity studies of some new fused 1,2,4-triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety. Eur. J. Med. Chem. 2009; 44:5066–5070.
- Fattah HAA. Synthesis, cytotoxic and antimicrobial activities of novel 1,2,4-triazole derivatives incorporating aryl sulfonamide moiety. Asian J. Pharma. Analysis and Medi. Chem. 2014; 2(3):145-162.
- Li X, Li XQ, Liu HM, Zhou XZ, Shao ZH. Synthesis and evaluation of antitumor activities of novel chiral 1,2,4-triazole Schiff bases bearing γ-butenolide moiety. Organic and Medicinal Chemistry Letters 2012; 2:1-5.
- Kumar GVS, Rajendraprasad V, Mallikarjuna BP, Chandrashekar SM, Kistayya C. Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010; 45:2063-2074.
- Godhani DR, Jogel AA, Sanghani AM, Mehta JP. Synthesis & biological screening of 1,2,4-triazole derivatives. Indian J. Chem. 2015; 54B:556-564.
- Plech T, Luszczki JJ, Wujec M, Flieger J, Pizo M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013; 60:208-215.
- Deng XQ, Wei CX, Li FN, Sun ZG, Quan ZS. Design and synthesis of 10-alkoxy-5, 6-dihydrotriazolo[4,3-d]benzo[f][1,4]oxazepine derivatives with anticonvulsant activity. Eur. J. Med. Chem. 2010; 45:3080-86.
- Wingrove PB, Wafford KA, Bain C, Whiting PJ, The Modulatory Action of Loreclezole at the γ- Aminobutyric Acid Type A Receptor is Determined by a Single Amino Acid in the β2 Subunit. Proc. Nat. Sci. USA, 1999; 91:4569-4573,.
- Jiang B, Huang X, Yao H, Jiang J, Wu X. Diaryl-1,2,4-Triazoles Bearing N-Hydroxyurea Moiety as Dual Inhibitors of Cyclooxygenase-2 and 5-Lipoxygenase. Org. Biomol. Chem. 2014; 12 (13),:2114-2127,.
- Murti Y, Agnihotri R, Pathak D. Synthesis, Characterization and Pharmacological Screening of Some Substituted 1,2,3- & 1,2,4-Triazoles. American J. Chem. 2011; 1 (2), 42-46.
- Suzgun PC, Basu NK, Basu A, Arora P, Talele TT, Durmaz I, Atalay RC, Kucukguzel SG. Anti-cancer and anti-hepatitis C virus NS5B polymerase activity of etodolac 1,2,4-triazoles. J.Enzyme Inhib.Med. Chem. 2015; 1-8.
| 32. Johns BA, Weatherhead JG, Allen SH, Thompson JB, Garvey EP, Foster SA, Jeffrey JL, Miller WH. The use of oxadiazole and triazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 1: Establishing the pharmacophore. Bioorg. Med. Chem. Lett. 2009; 19:1802-1806. |
- Dobbs KD, Feldman J, Marshall WJ, McLain SJ, Meth JS, Wang Y. Phosphorescent Iridium(III) Complexes of Cyclometalated 5-Aryl-1H- 1,2,4-Triazole Ligand Structural, Computational, Spectroscopic, and Device Studies. J. Phys. Chem. C. 2014; 118 (48):27763–27771.
- Hassan FA, Younus KW. Biological Evaluation of Some Azole Derivatives in Cooling Fluids (Lubricant Oils). Res. J. Bio. Sci. 2012; 7 (1):48-51.
