Changes in Nutritional Factors Affecting Tuberculosis: A Systematic Review
Amarachi Ruth Anigbo1, Elizabeth Kanayo Ngwu2, Manjinder Kour3*
1Research Scholar, School of Applied Sciences , Suresh Gyan Vihar University, Jaipur, India
2Professor, Department of Nutrition and Dietetics, University of Nigeria, Nsukka, Nigeria
3Assistant Professor, School of Applied Sciences , Suresh Gyan Vihar University, Jaipur, India
*Corresponding author, email: manjinderkour1992@gmail.com
Abstract
Nutrition plays a major role in the management and prevention of tuberculosis(TB); this happens in terms of the body immune response to the pathogenic organism. Nutrients like macro-nutrients and micronutrients, vitamins, proteins, carbohydrates are associated with boosting immune responses against pathogen mycobacterium tuberculosis causing tuberculosis. These nutrients have an immunomodulatory effects in controlling exposure to infectious agents, the process of inflammation and nutritional deficiency. When there is malnutrition, it increases the individual or group of population’s susceptibility to progressive stages from infection to disease. This review looks at various nutritional factors which includes the body mass index, weight, height, mid-arm circumference which are nutritional indicators that show when an individual is healthy or malnourished which leads to poor immune response that in turn affects an individual’s ability to fight mycobacterium tuberculosis infection or disease. Some researches have been carried out to show the relationship between nutritional factors and the roles they play in mycobacterium tuberculosis infection and prevention. But, very few evidences in the literature has shown a specific quantity of food that can alter the course of tuberculosis disease.
Keywords: Tuberculosis, Micronutrients, Macronutrients, Body mass Index, Mid Arm Circumference.
1. INTRODUCTION
Tuberculosis is a bacterial disease caused by Mycobacterium tuberculosis a condition in which the body oxidizes an abnormally large quantity of protein from foods rather than using them for protein synthesis and leading to a condition protein energy malnutrition which is a high-risk factor that exposes an individual or a group of persons to tuberculosis [1]. According to the World Health Organization (WHO), Tuberculosis has been ranked number 9 among the world leading cause of death [2], and these has been attributed to so many factors, but specifically to poor nutritional status especially among the regions classified as high burdened regions; of which India is ranked 5th among the list of 30 countries.
Nutrition can be defined as the science of eating food and the effects of food components on the metabolism, health, performance and disease resistance of human and animals. Food is good, but an adequate food is best for the immune system to function properly so as to be able to defend the entire body system when there are exposures to infection. For an individual or group of population to have adequate nutritional intake, the six classes of food which includes protein, carbohydrate, fats and oil, vitamins and minerals have to be taken in appropriate proportion. There is a very close relationship between nutrition and tuberculosis as deficiency of one or more nutrients in the body for a long period of time leads to the weakening of the immune system which pre-disposes the individual or group of population [3].
Nutritional indicators to show that a disease condition like tuberculosis is present includes body weight, height, body mass index, iron, calcium, magnesium levels, vitamin D, K, C, B12 levels. [4]. More so, when there is a pre-existing condition like human immunodeficiency virus (HIV), it plays a key role in enhancing the rate of infection while individual or group of population is exposed to TB transmission; this could be termed as exogenous factors. Certain factors like socioeconomic, environmental and behavioral are classified under exogenous factors as they play a vital role in determining the nutritional status of a population.
The objective of this study is to show nutritional factors that positively or negatively promote or prevent the occurrence of tuberculosis infection individually or in a group of population.
2. BODY MASS INDEX AND TUBERCULOSIS
Body mass index (BMI) plays a very important role in the well being of an individual or a population [5]. Based on the BMI an individual or group of individuals can be classified as underweight, normal and overweight Table1 [6]. A research conducted in rural China with adults; showed that BMI is associated with so many infections, and a low BMI is associated with a susceptibility to tuberculosis [7].
| Classification | BMI (kg/m2 ) |
| Healthy Weight | 18.5-24.9 |
| Overweight | 25-29.9 |
| Obesity I | 30-34.9 |
| Obesity II | 35-39.9 |
| Obesity III | 40 and above |
Table 1: Source: Adapted from WHO 1995, WHO 2000 and WHO 2004. Accessed 22/12/2017
BMI has also been shown as a major tool in determining the death rate among tuberculosis patient’s in China, It was observed that when there is malnutrition it leads to underweight in an individual or a group, that impairs or weakens the immune system [9]. Further more a study conducted in the United States showed that BMI, varied amongst races and gender still using the WHO BMI standards; Hispanic and men from other races (which includes the White/Caucasian, Black/African American, Middle eastern, Indian, native American, East-Asian, South-east Asian, pacific islanders) had a high BMI of 26kg/m2 and 25kg/m2 respectively and the females had a high body fat and a short height [10]. Undernutrition which results to a low BMI is one of the major problem faced by TB patients globally, especially in developing countries and it is was discovered in the study that one-third of the TB patients in Addis Abba health Centre were undernourished, and the prevalence of undernutrition among the adults TB patients was high [11].
3. THE ROLE OF VITAMINS IN TUBERCULOSIS
TB patients lack vital nutrients like vitamins, causing them to get malnourished, and placing them at a higher risk of getting infection due to weakened immune response. Vitamin as a dietary supplement has to be taken exogenously because human beings cannot synthesize vitamins or; if produced, the concentration can’t be sufficient. Human beings can synthesize Niacin (Vitamin B3) and Vitamin D but lack the ability to synthesize Thiamine (Vitamin B1), Riboflavin (Vitamin B2), Pantothenic Acid (Vitamin B5), Pyridoxine (Vitamin B6), Biotin (Vitamin B7), Folate (Vitamin B9), Cobalamin (Vitamin B12), Vitamin E, Vitamin C, and Vitamin K [12]. Nutritional supplements that has essential vitamins and minerals could help patients to fight against the disease by building up and strengthening their immune response [13]. Vitamins always have been considered an important supplement that boosts immunity. Recent studies have discovered the antimycobacterial nature of vitamins[14]. In one of the studies, it was discovered that Vitamin D possesses antimycobacterial properties which acts directly when added into a growth medium [15]. The same was also discovered for Vitamin A. Recently, a study by Vilchèze et al. [16] Showed that Vitamin C can inhibit M. tuberculosis through hydroxyl radicals produced in Fenton’s reaction. A common mechanism used by bactericidal antibiotics to cause cell death involves the generation of highly reactive hydroxyl radicals through fenton’s reaction. Hydroxyl radicals induce cell death by damaging the DNA, which is in part due to the oxidation of the guanine nucleotide pool.
In the presence of reductants like vitamin C in Fentons reaction, ferrous ions are produced by reduction of ferric ions. Therefore vitamin C has been discovered as a compound that speeds up fentons reaction which makes mycobacterium tuberculosis highly susceptible to killing. In a cross-sectional study carried out in Ethiopia, the concentration of Vitamin C, Vitamin E, and Vitamin A was found to be low in Tuberculosis patients as compared to the healthy controls [15]. Some reports states that maintenance of an adequate level of Vitamin D could be effective as a prophylactic method against some respiratory tract infections [17].
In addition, a close relationship has been found between Vitamin D levels and Tuberculosis [13]. In fact, in the advent of antibiotics, cod liver oil and exposure to sunlight was used for Tuberculosis treatment. Vitamin D activates production of cathelicidin in white blood cells to kill TB [18], Not only antimycobacterial properties of vitamins but also essential biosynthetic pathways operational in M. tuberculosis involving vitamins are being studied from the drug target perspective. Absence of corresponding pathways in human beings makes biotin and thiamin biosynthesis pathways attractive drug targets [19]. Vitamins play diverse role in the infection and pathogenicity of Tuberculosis. Studies involving thiamin, biotin, Vitamin C, and Vitamin D have gained significance primarily due to their potency as drug targets or because of their antimycobacterial properties [20].
A. VITAMIN B1
Vitamin B1 also known as thiamine is an essential micronutrient required for the proper functioning of amino acid and carbohydrate metabolic enzymes in its active form, i.e., thiamin diphosphate [21-22]. It is required for the biological activity of pyruvate dehydrogenase, transketolase, acetohydroxy acid synthase, and 2‑oxoglutarate dehydrogenase. M. tuberculosis does not have thiamin compensating mechanisms, making thiamin biosynthetic mechanisms an attractive drug target [23]. M. tuberculosis thiamin phosphate synthase, a gene involved in the synthesis of thiamin phosphate which is further phosphorylated to the final product, i.e., thiamin pyrophosphate in mycobacteria, was screened bioinformatically for drug targets by investigators. Results obtained after virtual screening were tested in vitro and one of the tested compounds showed potent antimycobacterial activity having a low inhibitory concentration (MIC) of 6 μg/ml [24]. Further studies are required to validate these findings and possibly reveal more potential drug targets.
B. VITAMIN B7
Vitamin B7 also called biotin is essential for growth and pathogenicity of M. tuberculosis. It works as a cofactor in two key enzymes required for fatty acid synthesis and anaplerosis namely acyl CoA carboxylase and pyruvate carboxylase [25]. These enzymes are responsible for the metabolic fixation of carbon dioxide. Biotin is an indispensable vitamin for all living organisms. However, its synthesis in plants, and some fungi is limited. Like thiamin, human beings are dependent on a dietary supplement and gut microflora for their daily uptake of biotin [26]. It has been suggested that de novo biotin biosynthesis is necessary for M. tuberculosis since it lacks biotin transporters as discovered by genetic studies. Moreover serum concentration of biotin is very small in human beings to meet the requirement needed [27]. Biotin is produced with the help of enzymes BioF, BioA, BioD, and BioB using pimeloyl‑CoA as a precursor [28-30]. Synthesis of biotin from pimeloyl‑CoA is well conserved in all the biotin producing organisms.
C. VITAMIN C
Vitamin C also called ascorbic acid is an essential micronutrient for human beings which has to be taken as a dietary supplement since humans cannot synthesize vitamin C because of the mutation in the gene encoding the enzyme gulonolactone oxidase [31]. Vitamin C protects its host from reactive oxygen and reactive nitrogen intermediates generated during mycobacterial infection [32]. For prevention of common cold and influenza, Linus Pauling in 1976 recommended 1–3 g/day of vitamin C. Several studies have suggested the role of vitamin C in the prevention and treatment of TB by oral administration the vitamin. Vitamin C deficiency has also been linked to TB infection [33]. A study conducted on a sample size of 1100 individuals diagnosed as not having tuberculosis initially, correlated their nutrition status with the susceptibility of Tuberculosis development. Of all individuals, 28 developed TB during the course of the study and it was observed that they had a less amount vitamin C concentration [34]. It has also been shown that Vitamin C acts as an activator for inducing dormancy in M. tuberculosis. Vitamin C induces DevR (DosR) regulation which is responsible for the development of dormancy in bacteria [35].
A study identifying antagonistic effects of vitamin C when used with rifampicin and isoniazid found some interesting results. It was observed that there was reduction in the colony forming units of wild type H37Rv strain as well as drug-resistant strains, when grown in the presence of vitamin C and rifampicin at substantial Minimum Inhibitory Concentration. Reduction in Colony Forming Unit in wild type and drug-resistant strains was also observed when vitamin C was tested together with isoniazid. However, isoniazid-vitamin C combination showed a weaker effect against resistant strains as compared to wild type H37Rv [36]. Narwadiya et al. have shown an association between vitamin C concentration and anti-TB properties of medicinal plants [37]. Vitamin C reduces ferric to ferrous ion which generates superoxide, hydrogen peroxide, and hydroxyl radicals in the presence of oxygen through Fenton and Haber–Weiss reaction [15]. These radicals damage the DNA and lipids of M. tuberculosis leading to growth impediment . Vitamin C is also believed to reduce the level of guanosine 5′-diphosphate-3′-diphosphate (ppGpp), a molecule thought to be involved in growth regulation and stress response in M. tuberculosis [38]. Recently it was observed that water soluble vitamins of which vitamin C is one, can prevent the progression of a disease causing organism because of its antioxidant activity. According to Khameneh et al. 2016, vitamin c has shown to selectively improve the antibacterial activity of anti-tuberculosis drugs against M.tuberculosis [39].
D. VITAMIN D
Vitamin D also called calciferol plays a role in maintaining calcium homeostasis and bone mineral density in human beings. It comes in two major forms namely ergocalciferol (Vitamin D2) and cholecalciferol (Vitamin D3). This Vitamin is obtained either through diet or exposure of epidermis to sunlight (ultraviolet B radiation-UVB). However, its role as a protective agent against various diseases is being researched [40]. Studies have found some relationship between vitamin D deficiency and its susceptibility to Tuberculosis since 1651, when the deficiency of vitamins was found to be associated with signs and symptoms of Tuberculosis for the first time. Later, heliotherapy became a common practice for patients with Tuberculosis and this formed the basis of treatment in sanatoria [41]. Meanwhile, Stead et al. showed racial differences in the incidence of Tuberculosis which was associated with the levels of 25 hydroxy Vitamin D [42]. Although conflicting results which emerged from clinical trials, in one of the studies carried out by Salahuddin et al., observed high doses of vitamin D supplementation which enhanced clinical and radiographic improvement in Tuberculosis patients [43]. In a contrast, studies carried out by Ralph et al.[44], and Daley et al.[45] observed no significant improvement in the culture conversion rate in vitamin D supplemented patients. There is a debate whether culture conversion rate can be a parameter while studying the effect of vitamin D supplementation. Tissue damage prevention may be an appropriate method during the study of vitamin D supplementation effects [46].
Reports has shown that vitamin D concentration in human beings depend on the season and latitude which indirectly has a relationship with Tuberculosis outbreak. A study carried out at Birmingham having data from 9739 patients over a period of 28 years pointed to the fact that Tuberculosis outbreaks increased by 24.1%, during the winters there is low incidence of sunshine with decreased vitamin D concentration which led to higher Tuberculosis outbreak in the winter season. A similar study was also conducted in South Africa having the same results [47]. Concentration of vitamin D in humans depends on various factors that include biosynthesis by human body, pigmentation, latitude, dietary supplementation, obesity, genetics, and disease status. Deficiency of vitamin D (<50 nmol/l 25(OH)D) is a global problem, and areas such as Middle East and South Asia have severe deficiency which in turn may increase the susceptibility to various diseases [48].
|
Vitamins Names |
Scientific Name | Solubility of Vitamins
|
Food Sources | |
|
Plant Sources |
Animal Sources | |||
| Vitamin A
|
Retinol | Fat Soluble | Riped yellow fruits, Yellow corn, Carrots, pumpkin, Squash, Spinach | Liver, Fish, Milk |
| Vitamin D | Cholecalciferol | Fat soluble | Mushrooms | Fish, Eggs, Liver |
| Vitamin B1 | Thiamine | Water Soluble | Oat meal, brown rice, potatoes | Eggs, Liver |
| Vitamin B7 | Biotin | Water Soluble | Leafy green vegetable, peanuts | Raw egg yolk, Liver |
| Vitamin C | Ascorbic acid | Water soluble | Fruits and vegetable | Liver |
Table 2: Showing the sources of vitamins discussed in the Review Article [49].
- PROTEIN-ENERGY MALNUTRITION AND TUBERCULOSIS
Proteins are large complex molecules that play an important role in the body. They perform mostly structural and regulatory function in the tissues and organs. Proteins are made up of thousands of smaller units called amino acids which have been found to have a link with tuberculosis infection. According to WHO, protein energy malnutrition is defined as an imbalance between the supply of protein and energy and the bodies demand for them to ensure optimal growth and function. In india, Protein Energy Malnutrition is one of the major concerns among other healths disorders because of its dire consequences ranging from physical to cognitive growth and susceptibility to infections [50]. The salvage cycle of infection and undernutrition go hand in hand. With inadequate dietary intake, the immune response gets weaker and increases susceptibility to infections [51]. A research study conducted by the University of California [UCLA] group of scientist discovered that protein play a key role in protecting people infected with Mycobacteruim tuberculosis from developing the active form of the disease. The protein interleukin-32 which is a protective protein was discovered to be one biomarker that has an adequate host defense against tuberculosis; it can induce killing of the TB bacterium only in the presence of vitamin D [52]. The in depth study of proteins and its interactions has given a clearer understanding with regards to tuberculosis disease through proteomics study [53]. Undernutrion is observed with patients who have tuberculosis infection and this is seen in the form of wasting which is decrease in the circulation of body protein mass, decreased fat mass and a reduced protein and energy intake. Protein energy malnutrition occurs as a result of insufficient protein essential for creating and regenerating body tissues; which greatly compromises the body’s immune functions [54]. Protein energy malnutrition could be seen in the following forms (i) acute malnutrition which is a precursor to wasting. (ii) chronic Malnutrition which leads to stunting [55]. Taking a critical observation at the feeding patterns or behaviour of a particular group of people; it can be deduced that their feeding behaviours or pattern goes a very long way in preventing malnutrition and infection like tuberculosis [56]. Poverty and income is more common to population with lower income and even if malnutrition is present in the upper income population, it is limited to the milder forms [57]. There is a disproportionate large number of impoverished and socially excluded groups in the society among the poor people, which exposes them to further deprivable poverty, food insecurity and undernutrition [58]. Though 26% of people live below the poverty line in India, 46% of under three children are suffering from undernutrition [59]. This shows that the prevalence of poverty solely cannot be responsible for undernutrition but is an underlying cause of factors like inadequate dietary intake, large family, infection, unhygienic environment and illiteracy which contributes to undernutrition among low income group and when they are undernourished, the immune system gets weaken which pre-exposes them to getting infected with tuberculosis.
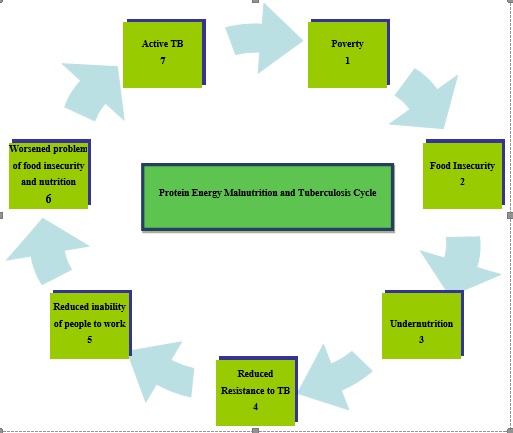
Figure 1: Showing the cycle relationship between Protein Energy Malnutrition and Tuberculosis
When there is no steady source of income or the income is very small, and the possibilities of getting a well paid job is highly competitive which is the case senario in the indian setting, food insecurity sets in, the people are not able to buy qualitative food that will be sufficient for the entire household. When these continue for a long period of time, undernutrition begins, members of the household begin to lack some vital nutrients that are required to build up the immune system; causing the immune system to be weak and become less resistant to primary infections like tuberculosis that will want to invade the imune system. Once the immune system is weak, the strength to work and look for sources to earn a living is greatly compromised which worsens the problem of food insecurity leading to a full disease(tuberculosis) as a result of lack of vital nutrients required by the body.
- MICRONUTRIENTS AND TUBERCULOSIS
Micronutrient deficiency happens as a result of increased metabolic demands and decreased intake of micronutrients which worsen the disease and delay recovery by supressing immune functions [60]. The following micronutrients have shown to be of great importance in improve immune response when there is tuberculosis infection; and these include Zinc, iron, copper, calcuim and selenium [61]. Zinc- Various studies on patients with tuberculosis had shown significantly lower plasma zinc level than those without tuberculosis, irrespective of their nutritional status. There was significant rise in zinc level at the end of six- months of antituberculosis therapy (ATT). Thus, it may be suggested that plasma zinc status is likely a marker for monitoring the severity of disease and response to therapy [62]. Zinc supplementation of patients with pulmonary tuberculosis helps to increase immunity and thereby speed up the recovery process [63]. An adequate supply of zinc needs to be ensured through foods such as seafood, meat, seeds, and cooked dried beans, peas and lentils [64]. Selenium- The essential trace element selenium has an important function in maintaining the immune processes and thus may have a critical role in clearance of the bacteria related to TB. Sea-foods and organ meats are the richest food sources of selenium. Other sources include muscle meats, cereals and other grains, and dairy products [65]. Anemia is highly prevalent among adults with pulmonary tuberculosis. In a study, concentration of hemoglobin was lower in tuberculosis patients than that in normal healthy subjects. There are two reasons for the association of low iron status and infection. One is that anemia which results from chronic infection and the other is that iron deficiency which increases the susceptibility of a person or group of individuals to infection like tuberculosis [66].
- CONCLUSION
In summary there is a very close relationship between macro nutrients and micro nutrients and the prevention, treatment and control of tuberculosis disease. Adequate nutrition plays a vital role in building up the immune system. If proper care and attention is paid to the classes of food nutrients we consume, this deadly disease tuberculosis will be a thing of the past. A healthy immune system is adequately fortified with food nutrients
7. REFERENCE
- Norman, J. T., Nelia, S. (2016). Community Nutrition for Developing Countries. AU Press, Athabasca University, Canada and Unisa Press, University of South Africa.
- World Global Report for Tuberculosis (2017) http://www.who.int/tb/publications/global_r/eport/en/ Accessed 17/11/2017
- https://www.collinsdictionary.com/dictionary/english/nutritionAccessed 27/11/2017
- Guoyao, W.U. (2016). Dietary protein intake and human health.Food Funct., 7(3), 1251-65
- Zhang, H., Li, X., Xin, H., Li H., Li, M., Lu, W., Bai ,L., Wang, X., Liu, J., Jin, Q., Gao, L. (2017). Association Of Body Mass Index With The Tuberculosis Infection: A Population-Based Study Among 17796 Adults In Rural China. Sci., Rep., 8(7), 41933.
- My idea
- Yen, Y. F., Chuang, P. H., Yen, M.Y., Lin, S. Y., Chuang, P., Yuan, M. J., Deng, C. Y. (2016). Association of Body Mass Index with Tuberculosis Mortality: A Population-Based Follow-Up Study. Medicine, 95(1), 2300-11.
- World Health Organization. (2018). Global Database on Body Mass Index http://apps.who.int/bmi/index.jsp?introPage=intro_3.html Accessed 30/09/2018.
- Cai, J., Ma, A., Wang, Q., Han, X., Zhao, S., Wang, Y., Schouten, E.G., Kok, F.J. (2017). Association between Body Mass Index and Diabetes Mellitus in Tuberculosis Patients In China: A Community Based Cross-Sectional Study. Public Health BMC, 17(1), 228-30
- Bennett, D. E., Courval, J. M., Onorato, I., Agerton, T., Gibson, J. D., Lambert, L., McQuillan, G. M., Lewis, B., Navin, T. R., Castro, K. G. (2008). Prevalence of Tuberculosis Infection in The United States Population: The National Health And Nutrition Examination Survey, 1999-2000. Am. J. Respir. Crit Care Med.177(3), 348-55.
- Berihun, D., Gezahegn, T., & Amare, W. (2016). Prevalence and Associated Factors of Undernutrition among Adult Tuberculosis Patients in Some Selected Public Health Facilities of Addis Ababa, Ethiopia: A Cross-Sectional Study. Nutrition BMC Series, 3(7), 1-9.
- Esposito, S., Lelii, M. (2015). Vitamin D and Respiratory Tract Infections in Childhood. BMC Infectious Diseases, 15, 487.
- Ströhle, A., Wolters, M., Hahn, A. (2011). Micronutrients at The Interface Between Inflammation and Infection – Ascorbic Acid and Calciferol. Part 2: Calciferol and The Significance of Nutrient Supplements. Inflamm. Allergy Drug Targets, 10(1), 64-74.
- Albahrani, A. A., & Greaves, R. F. (2016). Fat-Soluble Vitamins: Clinical Indications and Current Challenges for Chromatographic Measurement. The Clinical Biochemist Reviews, 37(1), 27–47.
- Vilchèze, C., Hartman, T., Weinrick, B., & Jacobs, W. R. (2013). Mycobacterium Tuberculosisis Extraordinarily Sensitive to Killing By a Vitamin C-Induced Fenton Reaction. Nature Communications, 1881(4), 1-10.
- Greenstein, R. J., Su, L., Brown, S. T. (2012). Vitamins A & D Inhibit The Growth Of Mycobacteria in Radiometric Culture. PLoS One, 7(1), e29631.
- Madebo, T., Lindtjørn, B., Aukrust, P., Berge, R. K. (2003). Circulating Antioxidants and Lipid Peroxidation Products in Untreated Tuberculosis Patients in Ethiopia. Am. J. Clin. Nutr., 78(1), 117‑223
- Patrick, J., McCullough, Douglas, S., Lehrer. (2018). Vitamin D, Cod Liver Oil, Sunshine, and Phototherapy: Safe, Effective and Forgotten Tools For Treating and Curing Tuberculosis Infections — A Comprehensive Review. J. Steroid Biochem. Mol. Biol., 177(1), 21-29.
- Combs, G. F. Jr., McClung, J. P. (2016). The Vitamins: Fundamental Aspects in Nutrition and Health. Cambridge, Massachusetts, United States: Academic Press.
- Du, Q., Wang, H., Xie, J. (2011). Thiamin (Vitamin B1) Biosynthesis and Regulation: A Rich Source of Antimicrobial Drug Targets. Int. J. Biol. Sci., 7(1), 41‑52.
- Salaemae, W., Azhar, A., Booker, G. W., Polyak, S. W. (2011). Biotin Biosynthesis in Mycobacterium Tuberculosis: Physiology, Biochemistry and Molecular Intervention. Protein Cell, 2(9), 691‑5.
- Settembre, E., Begley, T. P., Ealick, S. E. (2003). Structural Biology of Enzymes of the Thiamin Biosynthesis Pathway. Curr. Opin. Struct., Biol. 13(6), 739‑47.
- Khare, G., Kar, R., Tyagi, A. K. (2011). Identification of Inhibitors against Mycobacterium Tuberculosis Thiamin Phosphate Synthase, An Important Target For The Development of Anti‑TB Drugs. PLoS One, 6(7), e22441.
- Roje, S., (2007). Vitamin B Biosynthesis in Plants. Phytochemistry. Science Direct, 68(14), 1904‑21.
- Mock, D. M., Malik, M. I. (1992). Distribution of Biotin in Human Plasma: Most of The Biotin is Not Bound to Protein. Am. J. Clin. Nutr., 56(2), 427‑32.
- Hebbeln, P., Rodionov, D. A., Alfandega, A., Eitinger, T. (2007). Biotin Uptake in Prokaryotes by Solute Transporters with an Optional ATP‑Binding Cassette‑Containing Module. Proc. Natl. Acad. Sci. USA, 104(12), 2909‑14.
- Rodionov, D. A., Mironov, A. A., Gelfand, M. S. (2002). Conservation of the Biotin Regulon and the Bira Regulatory Signal in Eubacteria and Archaea. Genome Res., 12(10), 1507‑16
- Lin, S., Cronan, J. E. (2011). Closing in on Complete Pathways of Biotin Biosynthesis. Mol. Bio. Syst., 7(6), 1811‑21.
- Keer, J., Smeulders, M. J., Gray, K. M., Williams, H. D. (2000). Mutants of Mycobacterium Smegmatisimpaired in Stationary‑Phase Survival. Microbiology, 146(9), 2209‑17.
- Sassetti, C. M., Boyd, D. H., Rubin, E. J. (2003). Genes Required for Mycobacterial Growth Defined by High Density Mutagenesis. Mol. Micro. Bio., 48(1), 77‑84.
- Ogata, K., Izumi, Y., Tani, Y. (1973). The Controlling Action of Actithiazic Acid on The Biosynthesis of Biotin-Vitamers By Various Microorganisms. Agric. Biol. Chem., 37(5), 1079-85.
- Nishikimi, M., Yagi, K. (1996). Ascorbic Acid: Biochemistry and Biomedical Cell Biology. Subcell Biochem., 25(1), 17-35
- Andosca, J. B., Foley, J. A. (1948). Calcium Ribonate and Vitamin C (Nu 240-10) In the Treatment of Tuberculosis. Dis. Chest., 14(2), 107-14.
- Getz, H. R., Long, E. R., Henderson, H. J. (1951). A Study of the Relation of Nutrition to the Development of Tuberculosis. Influence of Ascorbic Acid and Vitamin A. Am. Rev. Tuberc. Pulm. Dis., 64(4), 381-93.
- Taneja, N. K., Dhingra, S., Mittal, A., Naresh, M., Tyagi, J. S. (2010). Mycobacterium Tuberculosis Transcriptional Adaptation, Growth Arrest and Dormancy Phenotype Development is Triggered By Vitamin C. PLoS One, 5(5), e10860.
- Khameneh, B., Fazly, Bazzaz, B. S., Amani, A., Rostami, J., Vahdati-Mashhadian, N. (2016). Combination of Anti-Tuberculosis Drugs with Vitamin C or NAC against DifferentStaphylococcus Aureus and Mycobacterium Tuberculosis Strains. Microb. Pathog., 93(1), 83-7.
- Narwadiya, S. C., Sahare, K. N., Tumane, P. M., Dhumne, U. L., Meshram, V. G. (2011). In VitroAnti-Tuberculosis Effect of Vitamin C Contents of Medicinal Plants. Asian J. Exp. Biol. Sci., 4(2), 2-22
- Mishra, A., Sarkar, D. (2015). Qualitative and Quantitative Proteomic Analysis of Vitamin C Induced Changes inMycobacterium Smegmatis. Front, Microbiol., 6(1), 451-6.
- Selvaraj, P., Harishankar, M., Afsal, K. (2015). Vitamin D: Immuno-Modulation and Tuberculosis Treatment. Can. J. Physiol. Pharmacol., 93(5), 377-84.
- Facchini, L., Venturini, E., Galli, L., de Martino, M., Chiappini, E. (2015). Vitamin D and Tuberculosis: A Review on a Hot Topic. J. Chemother., 27(3), 128-38.
- Stead, W. W., Senner, J. W., Reddick, W. T., Lofgren, J. P. (1990). Racial Differences in Susceptibility to Infection by Mycobacterium Tuberculosis. N. Engl. J. Med., 322(7), 422-7.
- Salahuddin, N., Ali, F., Hasan, Z., Rao, N., Aqeel, M., Mahmood, F.,et al. (2013). Vitamin D Accelerates Clinical Recovery From Tuberculosis: Results Of The SUCCINCT Study [Supplementary Cholecalciferol In Recovery From Tuberculosis]. A Randomized, Placebo-Controlled, Clinical Trial of Vitamin D Supplementation In Patients With Pulmonary Tuberculosis’. BMC Infect. Dis., 13(9), 22-24.
- Ralph, A. P., Waramori, G., Pontororing, G. J., Kenangalem, E., Wiguna, A., Tjitra E,et al. (2013). L-Arginine and Vitamin D Adjunctive Therapies in Pulmonary Tuberculosis: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS One, 8(8), e70032.
- Daley, P., Jagannathan, V., John, K. R., Sarojini, J., Latha, A., Vieth, R.,et al. (2015). Adjunctive Vitamin D For Treatment of Active Tuberculosis in India: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet. Infect. Dis., 15(5), 528-34.
- Cegielski, P., Vernon, A. (2015). Tuberculosis And Vitamin D: What’s The Rest of The Story? Lancet. Infect. Dis. 15(5), 489-90.
- Martineau, A. R., Nhamoyebonde, S., Oni, T., Rangaka, M. X., Marais, S., Bangani, N.et al. (2011). Reciprocal Seasonal Variation in Vitamin D Status and Tuberculosis Notifications in Cape Town, South Africa. Pro. Natl. Acad. Sci. USA 108(47), 19013-7.
- Edwards, M. H., Cole, Z. A., Harvey, N. C., Cooper, C. (2014). The Global Epidemiology of Vitamin D Status. J. Aging Res. Clin. Pract. 5(1), 148-58.
- https://en.wikipedia.org/wiki/Vitamin Assessed 08/1/2018
- https://ghr.nlm.nih.gov/primer/howgeneswork/protein accessed 21/12/2017
- National Nutrition Monitoring Bureau (NNMB) Diet and nutritional status of rural population, Technical report 21, India. 2002. [Last retrieved on 2010 Oct 03]. Available from: http://www.nnmbindia.org/NNMBREPORT2001.web.pdf . [Ref l
- Rachel Champeau | August 20, 2014 http://newsroom.ucla.edu/releases/study-identifies-protein-that-helps-prevent-active-tuberculosis-in-infected-patients. Accessed 21/12/2017
- https://proteomesci.biomedcentral.com/articles/10.1186/1477-5956-10-14. Accessed 21/12/2017
- Hood, M. L.H. (2013). A Narrative Review of Recent Progress in Understanding the Relationship Between Tuberculosis and Protein Energy Malnutrition. European Journal of Clinical Nutrition, 67(1), 1122-1128.
- World Health Organisation 2012. (cited 10 October 2012). Fact File: 10 Facts on Nutrition. Available from http://www.who.int/features/factfiles/nutrition/facts/ en/index.html4.
- Nutritional Status in Infancy and Early Childhood. (2008). [Last retrieved on 2010 Oct 02]. Available from: http://wcd.nic.in/research/nti1947/7.5%20iycn%203.2.2008%20prema.pdf
- Harishankar, Dwivedi, S., Dabral, S., B., Walia, D. K. (2013). Nutritional Status of Children Under 6 Years of Age. Indian J. Prev. Soc. Med., 35(1), 156–62.
- Cohen, M., Tirado, C., Aberman, N. L., Thompson, B. (2008). World Food Insecurity and Malnutrition: Scope, Trends, Causes and Consequences. Rome: International Food Policy Research Institute (IFPRI), Food and Agriculture Organization of the United Nations (FAO) 2008.
- Mendelson S, Chaudhuri S. Child malnutrition in India: Why does it persist? Child in Need Institute (CINI) [Last retrieved on 2010 Oct 07]. Available from: http://www.cini.org.uk/childmalutrition.pdf
- Shenkin, A. (2006). Micronutrients in Health and Disease. Postgraduate Medical Journal, 82(971), 559–567.
- https://blog.ekincare.com/2016/03/23/9-important-micronutrients-in-the-diet-of-tuberculosis-patients/ Accessed 08/1/2018
- Clinical Benefits of micronutrients in Tuberculosis. http://www4.dr-rath-foundation.org/research_news/articles/20140707_health_information_tuberculosis_edition20.html Accessed 008/1/2018.
- Tuberculosis and Nutrition.http://www.sun.ac.za/english/faculty/healthsciences/nicus/Documents/Files/Files/Fact_sheets/TB%20and%20Nutrition.pdf Assessed 08/01/2018
- https://www.eatforhealth.gov.au/food-essentials/five-food-groups/lean-meat-and-poultry-fish-eggs-tofu-nuts-and-seeds-and Assessed 08/01/2018
- Carolyn, D. B., Johanna, T. D., David, H. (2013). Handbook of Nutrition and FoodAssessed 08/1/2018utrition.s/s/Fdividualsberculosis. s infection;they are Zinc, iron, selenuim. worsen the disease and delay rec. CRC Press
- http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1806-37132014000400403 Assessed 08/1/2018
