Shelar PA1,2, Mishra A1
1 Suresh Gyan Vihar University, School of Pharmacy, Jaipur.
2Arvind Gavali College of Pharmacy, Jaitapur, Satara
ABSTRACT
Inflammation is one of the common events familiar to everyone and is body’s way to deal with infections and tissue damage. In Modern era, inflammation is one of the major reason for causing morbidity. A fine balance is maintained between inflammatory cascades and their potential for long term tissue damage and the imbalance causes acute as well as chronic debilitating diseases. Though mounting of inflammatory response is necessary for survival but the excessive and uncontrolled inflammation may leads to wide array of diseases like RA (rheumatoid arthritis), IBD (inflammatory bowel diseases), allergic asthma, allergic conjunctivitis, Crohn’s-disease, allergic rhinitis, chronic sinusitis, Diabetes, Alzheimer’s disease, cancer, atherosclerosis and cardiovascular diseases. Now a day, active constituents isolated from natural sources are very much important in development of new anti-inflammatory agents. However, different in-vivo and in-vitro animal models are available for evaluating anti-inflammatory activity of compounds. But for systemic evaluation of active constituents appropriate selection of animal model is important step. This review explain different models for assessing anti-inflammatory drugs including particular mechanism involved in inflammation, advantages, limitations of these models, and also mentioned the targeted compounds showing the anti-inflammatory effect. Hence, this present article provides a systemic approach for assessing the anti inflammatory activity of drugs can account for the identification of new chemical moieties during the early stage of screening of drugs.
Keywords: Anti-inflammatory, acute and chronic animal models, phytoconstituents, advantages, disadvantages.
INTRODUCTION
Vascular tissues show a biological response which is complex to harmful stimuli is known as inflammation. Harmful stimuli such as irritants, pathogens, chemicals and damaged cells are responsible to cause inflammation. Inflammation is shown by the organisms as a protective attempt to remove these harmful injurious stimuli and also initiate the process of healing for the tissues.1 However, inflammation process is needed for healing of wounds but if inflammation remains unchecked it leads to onset of different diseases like rhinnorhoea, atherosclerosis, and rheumatoid arthritis.2 Inflammation involves complex process of activation of enzymes, chemical mediators release, recruitment of fluid, cell migration, tissue damage and healing.3 It involves processes like protein denaturation, increased vascular permeability, also membrane alteration and are associated with swelling, pain, fever and redness4. Chemical mediators show a crucial role in inflammatory process that leads to inflammatory response. Mast cells, neutrophils, monocytes, macrophages and platelets binds to the specific receptors and causes blood vessels to permit the passage of substances (Vascular permeability), contraction of smooth muscles, neutrophil chemotaxis and various enzymatic activities. While the chemical mediators like leukotrienes, histamine (Vasoactive amines), prostaglandins, tumor necrosis factor, cytokines and serotonins are commonly causes the inflammation.
The cellular process of inflammation involves four steps:
- Changes in smooth muscle cell functioning leads to vasodilatation which leads to changes in blood flow,
- Cytoskeletal contraction in endothelial cell which alter vascular permeability,
iii. Phagocytic leukocytes get migrate towards site of inflammation.
- Phagocytosis. 1
Inflammation is of two types as acute or chronic. Pain, heat, erythma, primary loss of function and edema are the classical signs in the acute inflammation. The biologically active materials get released from lysosomal enzymes, polymorph nuclear leucocytes during the early inflammatory changes in the damaged or injured tissues.5 These active materials are responsible for initiation, progression, regulation and resolution of acute inflammation. This resolution is influenced by several anti-inflammatory agents and recruitment of monocytes which removes cell debris. This resolution generally not occur in acute phase but leads to chronic phase and such chronic inflammation is associated to different pathological conditions.6 Thus in acute inflammation increased vascular permeability occur which leads to infiltration and emigration of leukocytes while in chronic inflammation infiltration of macrophages, monocytes, nutrophils and mononuclear immune cells occur. After infiltration stimulation and fibroblast proliferation with fibrosis occurs.7
NECESSITY OF NEW ANTI-INFLAMMATORIES
Inflammation related disorders are affecting the numerous populations hence inflammatory diseases along with rheumatic diseases are serious health problem in the world. Though a lot many anti-inflammatory agents are available to treat number of inflammatory diseases, but their long term use leads to severe side effects.8 Steroidal (SAIDs) and non-steroidal (NSAIDs) agents are used in treatment of different inflammatory diseases9 where NSAIDs act by inhibiting cyclooxygenase (COX) and thus inhibit the biosynthesis of prostaglandins. As important role played by NSAIDs to reduce the consequences of inflammation but their long term use leads to renal, cardiovascular and gastrointestinal toxicities.10,11,12 While hyperglycemia, osteoporosis, growth arrest and hypertension occours due to chronic use of corticosteroids.13 Currently available treatment on discontinuation leads to toxicity and recurrence and is the major problem faced by the people.9 Hence, it is important to develop new anti-inflammatory agents which are safe and is a subject of great interest. 14
PLANT LEADS AS SOURCE OF ANTI-INFLAMMATORIES
From thousands of years throughout the world plants and their products have been used as basis of many traditional systems of medicine and still plants are used for treatment purpose as they provide new remedies. Plants contain different active compounds which offer great possibility in isolation, identification, estimation of these compounds and development of new drugs for treating inflammatory diseases.13 Phytochemicals are used in treatment of inflammation since the ancient time. The bark of Willow tree from 400 BC used as analgesic and antipyretic leads to the revelation of aspirin and in 1899 it is used as first potent drug in treatment of rheumatic diseases.15
ANTI-INFLAMMATORY PHYTOCONSTITUENTS- CURRENT STATUS AND SYSTEMIC APPROACH
Phytoconstituents are used as therapeutic agents from the centuries. Certain phytoconstituents like steroids, alkaloids, triterpenoids, flavonoids and phenols have potent anti-inflammatory properties. Phytoconstituents are the active moieties isolated from plants which are potent in their action hence these constituents’ shows therapeutic activities at micromolar concentration. As there are various advances in the allopathy, but still plant isolates are the sources of potential therapeutic agents not only in traditional systems but also in modern system of medicine.13 That means the plant extracts and their isolated compounds contribute to the drug revelation and developing new pharmaceuticals for their use in humans.16
ANIMAL MODELS FOR EVALUATION OF ANTI-INFLAMMATORY ACTIVITY
Specific models are used or designed to search new chemical moiety from nature and also to study the mechanism by which these moieties are showing action. Pharmacology plays an vital role in searching such moieties as it provides different models which are clinically and physiologically relevant to human beings before start of any experimental assay it is necessary to plan that experiment by considering sample size, route of administration, statistical methods and also use of positive control.17 Different designed experimental models are used to evaluate the inflammation by means of animal and biochemical models of inflammation. 18
There are two broad classes of experimental models:
- Acute inflammatory models
- Chronic inflammatory models
Acute models are used to test the drugs which modulate erythema (blood flow), leukocyte migration, Changes in blood vessel permeability, phagocytosis, chemotaxis, antipyretic, local analgesic action, paw edema while chronic models are used to find the drugs that harmonize the disease process and it includes granuloma pouches, pellet and sponge implants, adjuvant induced arthritis. 18 These models are widely used specially the rat paw edema test. These models can be utilized in numerous ways by using different inflammatory agents.
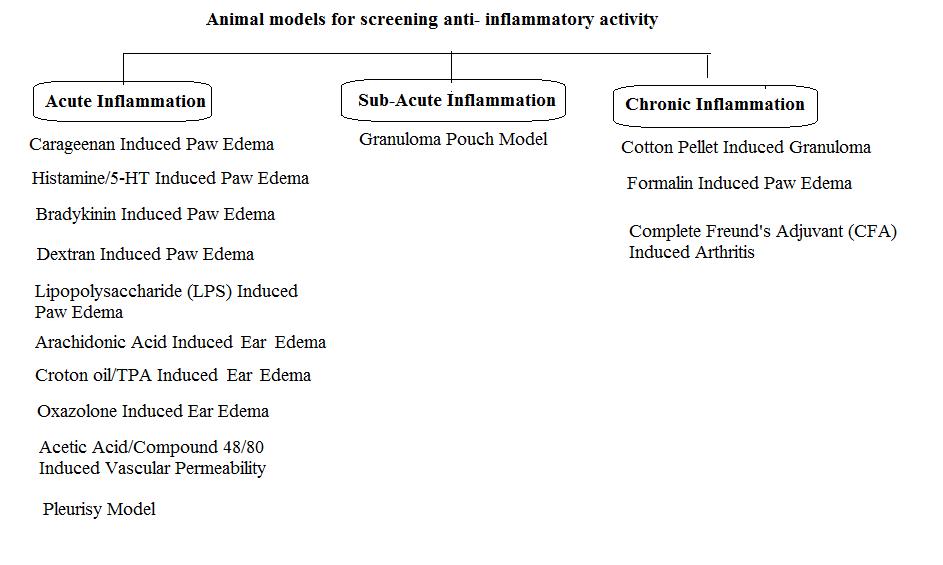
Figure 1: Animal models for screening anti-inflammatory activity.
ACUTE INFLAMMATORY MODELS
Carrageenan induced paw edema:
This model is commonly and majorly used for anti-inflammatory activity for plant derived as well as synthetic compound.19,20 This model is distinctive with more reproducibility for acute inflammation.21 The activation of complementary system and mediators get activated by sulphated sugar present in carrageenan22 At the initial phase of inflammation carrageenan stimulate phospholipase A2 while the progress the inflammation occur due to cytotoxic effects.23 This model causes activation of cyclo-oxygenase pathway. The edema produced by carrageenan shows biphasic curve.24 Where the first phase is partly related to injection trauma and releases serotonin and histamine which act as acute phase mediators.25 While during the second phase, prostaglandins are important and this takes about three hours for development of inflammation after carrageenan injection.
In the initial phase of inflammation following mechanism occurs:
- Carrageenan injection
- Dilates post capillary venules
- leads to exudation of fluid and cells
- Releases pro-inflammatory mediators
Advantages:
- It is the most preferred model of inflammation as the inflammation produced is reproducible, acute and non-immune. 21
- Multiple mechanism are involved which makes this model as preliminary choice for screening anti-inflammatory agents.
- As the biphasic response is produced, it allows this model to anticipate biological targets that are probable for test drug in the inflammation.21
- This model is suitable for testing the drugs that inhibit cyclo-oxygenase which is necessary for prostaglandin synthesis. Eg. NSAIDs.21
Limitations:
- This model needs at least one week for acclimatization of animals before start of an experiment.
- Trained persons are needed to investigate the activity for recording stable and reproducible results and also the type and preparation of solution needs careful attention 26, 27
Histamine/5-HT induced Paw Edema:
Histamine causes acute inflammation and is used for testing various anti-inflammatory drugs. Histamine and 5 HT act by increasing vascular permeability and they act by activation of prostaglandins which produces inflammation.28, 29, 30 The administration of histamine stimulates the H1 receptors which causes contraction of endothelial cells which disrupt the endothelial barrier hence result into increased blood flow in to extracellular spaces those results into development of edema.30,31,32 Histamine also secrets prostaglandins and neuropeptides, causes inflammation and hyperalgesia.33 Similarly 5-HT also increases vascular permeability by means of introducing endothelial gaps.34
Advantages:
- It is suitable method for assessing the performance of acute inflammatory effects of substances.
- These models are used to for testing the drugs that shows anti-inflammatory action by inhibition of histamine or 5-HT.
- These models are used as second authorized models for the drugs that showed activity at initial phase of Carrageenan induced inflammation, to analyze the results of carrageenan induced paw edema.34, 35
Disadvantages:
- The inflammation produced by histamine and 5-HT is less and temporary.
- These models are not suitable for appraising the drugs act as prostaglandin inhibitor as the act by means of mechanism other than histamine or 5-HT.36
Bradykinin-induced Paw Edema:
Prostaglandins (PGs) are responsible for bradykinin-induced paw edema where bradykinin stimulate phospholipase which promotes biosynthesis of PGs (prostaglandins). Also release of metabolites of arachidonic acid when endothelial cell cultures of human are incubated with bradykinin.37
Advantages:
- This model is used for acute inflammation.
- This is a proper model for prostaglandin inhibiting drugs.
Disadvantages:
- The edema produced by bradykinin is temporary and mild.37
Dextran-induced Paw Edema:
In this model administration of dextran releases mediators like histamine and serotonin via increased vascular permeability and kinins activation where these mediators get interact with respective receptors (such as H1, H2 and 5HT2) which develops the osmotic edema having minimal neutrophils and proteins. This model leads to development of fast and short lived edema.38, 39, 40
Advantages:
- As this model develops edema by releasing of histamine and serotonin hence, this model is used to evaluate the anti inflammatory activity of drugs having anti-histaminic or anti-serotonin property.
- It is also used to analyze the results obtained by paw edema induced by carrageenan.
Disadvantages:
- This model is not suitable for the drugs showing anti-inflammatory effect other than anti-histaminic and anti-serotonin like mechanism.38, 39
Lipopolysaccharide (LPS)-induced Paw edema:
Injection of LPS produces acute inflammation as well as swelling if injected rat paw. LPS causes rise in the IL-1β, TNF-α as well as myeloperoxidase activity in mouse paw. Hence, paw edema introduced by LPS is used to identify drugs that reduce inflammation by acting against TNF-alfa.41, 42
Advantages:
- Lipopolysaccharides not only produces inflammation but they also shows inflammatory hyperalgesia hence this model is simultaneously used for evaluation of anti-inflammatroy as well as analgesic activity.41
- This model is appropriate for testing the drugs that shows anti-inflammatory effect via modulation of cytokine.
Arachidonic acid-induced ear edema:
This model is mainly used for evaluating the potential of natural extracts as well as synthetic compounds as anti-inflammatory agents.43 Cutaneous inflammation produced by arachidonic acid gives valuable information of anti-inflammatory agents that are used in treatment of skin inflammation.44 Application of AA on particular are of skin causes inflammation by means of eicosanoids like leukotriene C4 (LTC4), prostaglandin-E2 (PGE2) and thromboxanes. This eicosanoids cause release of histamine by means of mast cell degranulation hence in this model the anti-inflammatory activity shown by the compounds is corresponds to antioxidant and antihistaminic property of the compound.45 This inflammation is demonstrated as edema, intense erythema and accumulation of neutrophills. 46
Advantages:
- This model is appropriate for topical acute inflammation.
- It is used to identify the anti-inflammatory agents that act through eicosanoids inhibition.
Disadvantages:
- At the completion of protocol animals are sacrificed.43, 47
Croton oil/ TPA-induced Ear Edema:
Croton oil or its principle (irritant) (12-O-tetradecanoylphorbol-13 acetate) causes ear edema and are used to evaluate anti-inflammatory action of non-steroidal and steroidal anti-inflammatory drugs.44 Herbal extracts as well as synthetic anti-inflammatory compounds are screened by means of this model.48 TPA model is used to study synthetic and local anti-inflammatory compounds.49 Vasodilation is caused by topical use of croton oil which increases permeability of blood vessels resulting into influx of neutrophil that produces eicosanoids which releases histamine and serotonin.45 The cyclo-oxygenase and lipo-xygenase inhibitors shows inhibition of TPA induced inflammation.48 While the TPA causes protein kinase C activation which is responsible for activation of MAPKs (Mitogen-activated protein kinase) as well as phospholipase A2 Where phospholipase A2, COX and LOX inhibitors as well as corticosteroids reduces inflammation occoured due to TPA.45,14
Advantages:
Steroidal and non steroidal anti inflammatory drugs can be screened properly by using this model.
In this model more potent results are correlated with COX inhibitors and less potent results are correlated with LOX inhibitors.
Disadvantages:
The model shows multiple mechanisms hence it is used to predict the mode of action of anti-inflammatory drugs but not for approving their actions.
Animals are generally sacrificed after experimentation for further investigation 44, 45
Oxazolone induced ear edema:
Oxazolone when applied topically it leads to increase in prostaglandin and leukotrienes like metabolites of arachidonate in tissues. It also shows increase in NOS-2 (Nitrous oxide synthetase -2) in keratinocytes. Oxazolone causes skin sensitization by increasing CD-8 + T-lymphocytes. Oxazolone act as allergen and it initiate DTH (delayed type hypersensitivity). Inhibition of DTH induced by oxazolone and control on eicosanoids levels occurs due to inhibitors of cortico-steroids and specific cytokine expression.50,51 In the experimental animals chronic contact dermatitis can be induced by repeated application of oxazolone to the ears, this chronic dermatitis is represented by noticeable inflammatory cell infiltration, continued ear swelling and remarkable epidermal hyperplasia. Additionally there occour marked rise in IFN-ɤ (interferon-ɤ) level and small variation in IL-4 level where interferon-ɤ causes activation of various inflammatory cells which increases keratinocytes proliferation and causes thickening of epidermal cells.52, 53
Advantages:
- It is significant model for immune inflammation.
Disadvantages:
- Drugs that show anti-inflammatory effect only through non-immune mechanisms may get excluded.
- In this method after completion of experiment animals are need to be sacrificed.
Acetic acid/ compound48/80-Induced vascular permeability:
Compound 48/80 is powerful activator of histamine release by means of degranulation of mast cell.54 At the time of inflammation it causes arterioles and venules dilation by means of mast cells stimulation which causes release of mediators like prostaglandins, histamine and leukotrienes and thus increases vascular permeability. Due to this increased vascular permeability plasma constituents (antibodies and complement) get access towards the tissues that are infected or injured.55 Due to raised vascular permeability and subsequent edema the plasma proteins and fluids force out from the vessels into the surrounding area. The assessment of increased blood vessel permeability is carried out by using Evan’s blue dye at injected sites.56
Advantages:
- It is suitable model for acute anti-inflammatory effect.
- The anti-inflammatory agents which show activity against increased vascular permeability caused by compound 48/80 are linked with antihistaminic activity or mast cell stabilization of drugs.
Disadvantages:
- Severe irritation is caused by acetic acid when injected through intraperitoneal route and is inappropriate with respect to animal welfare.
- Often, after completion of experiment, animals are sacrificed.56, 55
Pleurisy Model:
In the experimental animals pleurisy is induced by different inflammatory agents like dextran, compound 48/80, enzymes, carrageenan and antigens. These agents cause exudative inflammation. Pleurisy induced by carrageenan causes acute inflammation and the parameters like leukocyte migration, fluid blowout, different biochemical parameters are evaluated.56, 57 The quantitative assessment of pleural exudates, leukocyte migration inhibition and total protein content represents the anti-inflammatory response of test drug which is acute.58
Advantages:
- This is significant model for acute inflammation.
- This model enables to assess inflammatory phenomenon like leukocyte migration, fluid extravasation and biochemical-parameters in exudates.
Disadvantages:
- This method imposes severe pain and accompanied by systemic infection.
- Generally animals are sacrificed after the completion of experiment.56, 57
SUB-ACUTE INFLAMMATION MODELS
Granuloma Pouch Model:
The rapid multiplication of granulation tissue occurs when irritant substances are administered into the subcutaneous air pouch. Additionally, infiltration of macrophage and poly-morphonuclear leukocyte occurs due to administration of irritant substances. Sometimes there is chance of exposure of growing tissues to mutagenic and carcinogenic substances. Direct contact of test drugs facilitated with the target cells when test drug is administered in the form of air pouch.56
Advantages:
- This model is used to study sub-acute inflammation.
- Direct contact of test compounds with target cells occur when test drug is administered in the form of air pouch.
Disadvantages:
- This method is painful in the experimental animals.
- Anesthesia is given during procedures.
- Frequently animals need to be sacrificed after completion of protocol.56, 58
CHRONIC INFLAMMATION
Cotton Pellet-Induced Granuloma:
This model is commonly used for evaluation of chronic inflammation as it shows characteristic pathological events similar to that occur in chronic inflammation. This model is commonly used for assessing newer compounds.40 It shows fibroblast proliferation, monocyte infiltration, angiogenesis and exudation.59 The proliferation of macrophases, neutrophils and macrophases along with multiplication of small blood vessels produce granulomatous tissue that is highly vascularized reddish mass and this granulomatous tissue is characteristic of chronic inflammation.60 In this method, the cotton pellet absorbs the fluid which affects the moist weight of granuloma. The moist weight of cotton pellet is related with moist transudate while the dry weight is linked with granulomatous tissue formation.61 For this model corticosteroids are found to be effective as they show their action at proliferative stage and inhibit the inflammation.20
Advantages:
- This model is commonly used for chronic inflammation.
- The moist weight of granuloma is related to amount of transudate while the dry weight of granuloma is related with granulomatous tissue formation.
- The chronic irritation and inflammation proliferative changes occur where the granuloma’s biochemical analysis of gives information regarding these proliferative changes.
- This model is used as for assessing newer anti-inflammatory drugs.
Disadvantage:
- In this method localized sepsis occurs due to implantation which leads to dumbfound observations.
- This method anaesthetic and surgical skills.
- Removing surgical stitches, repeated handling of animals and sacrifice the animals are the limitations of this model.20, 61
Formalin induced paw edema:
This model is used for assessing chronic anti-inflammatory effect of different drugs. This model is closely related with human arthritis.62 Formalin induces biphasic inflammation where the early phase is neurogenic phase conciliated by substance-P and bradykinin. While the later phase shows involvement of prostaglandins, 5-HT, histamine and bradykinin.54 The drugs like opioids suppress both phases while the drugs like NSAIDs and corticosteroids inhibit the second phase.63
Advantages:
- This model is having close resemblance to the human arthritis.
- It is commonly used for chronic inflammation.
- This model helps to study involvement of central or peripheral components in the drug’s anti-inflammatory effect.
Disadvantages:
- Formalin is severe irritation causing agent hence the experimental animals faces severe pains after administration of formalin.63, 54
Complete Freund’s Adjuvant (CFA) Induced arthritis:
This model shows chronic inflammation where in experimental animals it causes synovial hyperplasia including several systemic changes.64 Inflammation results from enormous leukocyte infiltration, increase in levels of chemokine and cytokine along with IL-1β and TNF-α, cartilage and bone destruction, release of ROS also causes swelling and deformation.64, 65, 66 Injection of CFA in to rat footpad causes inflammation of ligaments and joints. In the initial phase of CFA inflammation, edema is produced and it increases gradually and within two weeks it remains constant. The anti-inflammatory effect was studied by measuring injected and non-injected paw edema along with antioxidant estimation, biochemical and hematological evaluations with radiological and histo-pathological study, visual arthritis scoring, nitrite content determination helps to know the possible mechanisms for anti-inflammatory as well as analgesic effects of tested compounds.67, 68
Advantages:
- This model is used for chronic inflammation and for arthritic alteration.
- Inflammation produced by CFA involves immune inflammatory components.
- CFA induced inflammation shows primary and secondary lesions and these resemble to the clinical symptoms of human inflammation and arthritis, respectively.
- The test drugs can be tested against acute, chronic, immune mediated inflammation and arthritic conditions by using this model.
Disadvantages:
- Administration of CFA need careful attention as it affects the arthritic severity response.
- The time required for activity is more.
- Induction of CFA, exposes experimental animals to painful condition.
- For evaluation of inflammatory paw volume it requires plethysmometer and von Frey apparatus.
- After completion of protocol animals are need to be sacrificed.64, 67
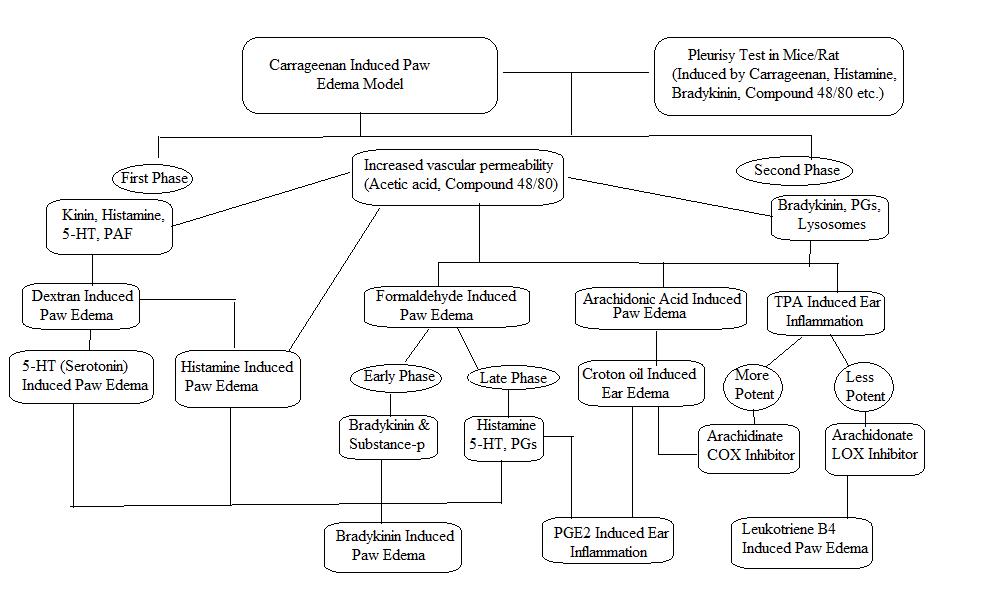
Figure 2: Scheme for preclinical evaluation of acute anti-inflammatory activity.
CONCLUSION
Plants consist of different secondary metabolites which are responsible for different pharmacological actions. Recently, natural sources are found to be of great interest in the process of discovery of drug where the use of plant isolates to study anti-inflammatory activity and also other activities have been increased. These plant isolates offer a hope for finding the potent anti-inflammatory compounds. These plant constituents show anti-inflammatory activity by acting on different inflammatory targets. Also they show the action at minute concentration and therefore reduce the risk of side effects. But, study of these phytoconstituents is not carried out till the determination of molecular mechanism also their pharmacokinetics. Hence, the systematic study of these phytoconstituents using suitable animal models, estimation of biochemical parameters, molecular estimation, pharmacokinetic study and also safety data, etc should be studied to find the new anti-inflammatory agents from natural sources.
BIBILOGRAPHY
- Denko CW. A role of neuropeptides in inflammation, In: Biochemistry of Inflammation. Kluwer Publisher; London, 1992, 177-181.
- Henson PM, Murphy RC. Mediators of Inflammatory Process. Amsterdam Elsevier; 1989, 404.
- Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res. 1995;44(1):1-10.
- Umapathy E, Ndebia EJ, Meeme A, et al. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plants Res. 2010;4(9):789-795.
- Barbosa-Filho JM, Piuvezam MR, Moura MD, et al. Anti-inflammatory activity of alkaloids: a twenty-century review. Rev Bras Farmacogn. 2006;16(1):109-139.
- Ahmed AU. An overview of inflammation: Mechanism and consequences. Front Biol China. 2011;6(4):274-281.
- Grover S, Tandon S, Misra R, Aggarwal A. Interleukin-1 receptor antagonist gene polymorphism in patients with rheumatoid arthritis in India. Indian J Med Res ·. 2006;123(June 2006):815-820.
- Divya TS, Latha PG, Usha K, et al. properties of Wattakaka volubilis ( Linn . f .) Stapf . Nat Prod radiance. 2009;8(2):137-141.
- Jo WS, Yang KM, Choi YJ, et al. In vitro and in vivo anti-inflammatory effects of pegmatite. Mol Cell Toxicol. 2010;6(2):195-202.
- Sofidiya MO, Imeh E, Ezeani C, Aigbe FR, Akindele AJ. Antinociceptive and anti-inflammatory activities of ethanolic extract of Alafia barteri. Brazilian J Pharmacogn. 2014;24(3):348-354.
- Kumari KDKP, Weerakoon TCS, Handunnetti SM, Samarasinghe K, Suresh TS. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J Ethnopharmacol. 2014;151(3):1202-1208.
- De Oliveira RG, Mahon CPAN, Ascêncio PGM, Ascêncio SD, Balogun SO, De Oliveira Martins DT. Evaluation of anti-inflammatory activity of hydroethanolic extract of Dilodendron bipinnatum Radlk. J Ethnopharmacol. 2014;155(1):387-395.
- Gautam R J. Recent Developments in Anti- Infammatory Natural Products. Med Res Rev. 2009;29(5):767-820.
- Gorzalczany S, López P, Acevedo C, Ferraro G. Anti-inflammatory effect of Lithrea molleoides extracts and isolated active compounds. J Ethnopharmacol. 2011;133(3):994-998.
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231(25):232-235.
- Lahlou M. The Success of Natural Products in Drug Discovery. Pharmacol & Pharm. 2013;04(03):17-31.
- Butterweck V, Nahrstedt A. What is the best strategy for preclinical testing of botanicals? A critical perspective. Planta Med. 2012;78(8):747-754.
- Lewis DA. Anti-Inflammatory Drugs from Plant and Marine Sources. Birkhauser Verlag; Basel, 1989.
- Boominathan R, Parimaladevi B, Mandal SC, Ghoshal SK. Anti-inflammatory evaluation of Ionidium suffruticosam Ging. in rats. J Ethnopharmacol. 2004;91(2-3):367-370.
- Panthong A, Norkaew P, Kanjanapothi D, Taesotikul T, Anantachoke N, Reutrakul V. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. J Ethnopharmacol. 2007;111(2):335-340.
- Sarkhel S. Evaluation of the anti-inflammatory activities of Quillaja saponaria Mol. saponin extract in mice. Toxicol Reports. 2016;3:1-3.
- Osadebe PO, Okoye FBC. Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J Ethnopharmacol. 2003;89(1):19-24.
- García MD, Fernández MA, Alvarez A, Saenz MT. Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae). J Ethnopharmacol. 2004;91(1):69-73.
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw. Exp Biol Med. 1962;3(111):544-547.
- Perianayagam JB, Sharma SK, Pillai KK. Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J Ethnopharmacol. 2006;104:410-414.
- Morris CJ. Carrageenan-Induced Paw Edema in the Rat and Mouse. Vol 225. Springer Science and Business Media LLC; 2003.
- Whiteley PE. Models of Inflammation : Carrageenan-Induced Paw Edema in the Rat.; Current protoc. Pharmacol. 1998, 5-6
- Vasudevan M, Gunnam KK, Parle M. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. J Ethnopharmacol. 2007;109:264-270.
- Singh B, Bani S, Gupta DK, Chandan BK, Kaul A. Anti-inflammatory activity of ‘ TAF ’ an active fraction from the plant Barleria prionitis Linn . J Ethnopharmacol. 2003;85:187-193.
- Ben IO Anti-inflammatory effects of Napoleona imperialis P . Beauv . ( Lecythidaceae ) on rat model of inflammation. Indian J Heal Sci. 2016;9(1):89-95.
- Kumar V, Abbas A.K. Robbins and Cotran Pathologic Basis of Disease. Elsevier Health sciences; London, UK, 2014.
- Hussain A A, Zeitlin IJ. RE-APPRAISAL OF THE ROLE OF HISTAMINE IN CARRAGEENAN-INDUCED PAW OEDEMA. Eur J Pharmacol. 1983;88:169-176.
- Eddouks M, Chattopadhyay D, Zeggwagh NA. Animal models as tools to investigate antidiabetic and anti-inflammatory plants. Evidence-based Complement Altern Med. 2012;2012:1-14.
- Raud J, Konrad D. Delayed anti-inflammatory action of nedocromil sodium in the rat paw is dependent on de novo protein synthesis. Eur J Pharmacol. 1995;282:207-211.
- H. N. Early and delayed phases of hind paw edema in rats. Japan J Pharmacol. 1974;24:393-405.
- Cole HW BC. Serotonin-induced Paw Edema in the Rat : Pharmacological Profile. Gen Pharmacol. 1995;26(2):431-436.
- Vilar Marina, Bark AL, Suênia DA, Souza GL De, et al. Assessment of Phenolic Compounds and Anti-Inflammatory Activity of Ethyl Acetate Phase of. Molecules. 2016;21:1-17.
- Okoli CO, Akah PA. Mechanisms of the anti-inflammatory activity of the leaf extracts of Culcasia scandens P . Beauv ( Araceae ). Pharmacol Biochem zbehavior. 2004;79:473-481.
- Coura CO, Souza RB, Ariévilo J, Rodrigues G. Mechanisms Involved in the Anti- Inflammatory Action of a Polysulfated Fraction from Gracilaria cornea in Rats. PLoS One. Published online 2015:1-18.
- Babu NP, Pandikumar P, Ignacimuthu S. Anti-inflammatory activity of Albizia lebbeck Benth ., an ethnomedicinal plant , in acute and chronic animal models of inflammation. J Ethnopharmacol. 2009;125:356-360.
- Calil IL, Zarpelon AC, Guerrero ATG, et al. Lipopolysaccharide Induces Inflammatory Hyperalgesia Triggering a TLR4 / MyD88-Dependent Cytokine Cascade in the Mice Paw. PLoS One. 2014;9(3):1-8.
- Vajja BNL, Juluri S, Kumari M, Kole L, Chakrabarti R, Joshi VD. Lipopolysaccharide-induced paw edema model for detection of cytokine modulating anti-inflammatory agents. Int Immunopharmacol. 2004;4:901-909.
- Tamura Koji E, Spada R, Waismam K, et al. Inhibitory effects of Solidago chilensis Meyen hydroalcoholic extract on acute inflammation. J Ethnopharmacol. 2009;122:478-485.
- Inoue H, Mori T, Shibata S, Koshihara Y. Modulation by glycyrrhetinic acid derivatives of TPA-induced mouse ear oedema. Br J Pharmacol. 1989;96:204-210.
- Boller S, Soldi C, Marques MCA, et al. Anti-inflammatory effect of crude extract and isolated compounds from Baccharis illinita DC in acute skin inflammation. J Ethnopharmacol. 2010;130:262-266.
- Nonato Regina F, Magalhães T, Nogueira O, Adelita T, Barros DA. Antinociceptive and antiinflammatory activities of Adiantum latifolium Lam .: Evidence for a role of IL-1 β inhibition Antinociceptive and antiinflammatory activities of Adiantum latifolium Lam .: Evidence for a role of IL-1 inhibition. J Ethnopharmacol. 2011;136:518-524.
- Moreno JJ. Effect of aristolochic acid on arachidonic acid cascade and in vivo models of inflammation. Immunopharmacology. 1993;26:1-9.
- Bralley EE, Greenspan P, Hargrove JL, Wicker L, Hartle DK. Topical anti-inflammatory activity of Polygonum cuspidatum extract in the TPA model of mouse ear inflammation. J Inflamm. 2008;7:1-7.
- Sadeghi H, Parishani M, Touri MA, et al. Pramipexole reduces inflammation in the experimental animal models of inflammation. Immunopharmacol Immunotoxicol. Published online 2017:1-7.
- Bas, Giner RM, Cerd M. Inhibition of the pro-inflammatory mediators ’ production and anti-inflammatory effect of the iridoid scrovalentinoside a n. J Ethnopharmacol. 2007;110:419-427.
- Bas E, Recio MC, Máñez S, et al. New insight into the inhibition of the inflammatory response to experimental delayed-type hypersensitivity reactions in mice by scropolioside A. J Ethnopharmacol. 2007;555:199-210.
- Fujii Y, Takeuchi H, Tanaka K, Sakuma S, Ohkubo Y, Mutoh S. Effects of FK506 ( tacrolimus hydrate ) on chronic oxazolone-induced dermatitis in rats. Eur J Pharmacol. 2002;456:115-121.
- Barkar JN, Allen. The effect of In-vivo interferon gamma on the distribution of LFA-1 and ICAM-1 in normal human skin. J Invest Dermatol. 1989;93(4):439-444.
- Shuichi S, Takata Y, Kaneda H, Watari J. Effects of a Hop Water Extract on the Compound 48 / 80-Stimulated Vascular Permeability in ICR Mice and Histamine Release from OVA-Sensitized BALB / c Mice. Biosci Biotechnol Biochem. 2007;71(6):1577-1581.
- Li C, Wu X, Zhao X, et al. Anti-Inflammatory Property of the Ethanol Extract of the Root and Rhizome of Pogostemon cablin ( Blanco ) Benth. Sci World J. 2013;2013:1-11.
- Patel M, K SG. In Vivo Animal Models in Preclinical Evaluation of Anti- Inflammatory Activity- A Review. Int J Pharm Res Allied Sci. 2012;1(2):1-5.
- Rachmawati H, Safitri D, Pradana AT, Adnyana IK. TPGS-Stabilized Curcumin Nanoparticles Exhibit Superior Effect on Carrageenan-Induced Inflammation in Wistar Rat. Pharmaceutics. 2016;8(24):1-13.
- Wu Y, Zhou C, Song L, et al. Effect of total phenolics from Laggera alata on acute and chronic inflammation models. J Ethnopharmacol. 2006;108:243-250.
- Meshram Gulab, Anil K, Rizvi W, Tripathi CD, Khan RA. Journal of Traditional and Complementary Medicine Evaluation of the anti-in fl ammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. J Tradit Chinese Med Sci. Published online 2015:1-4.
- Amresh G, Reddy GD, Rao C V, Singh PN. Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol. 2007;110:526-531.
- Gupta M, Mazumder UK, Kumar RS, et al. Anti-inflammatory , analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J Ethnopharmacol. 2005;98:267-273.
- Juma Mahmood K, Ahmed ZA, Numan IT, Abdul S. Dose-dependent anti-inflammatory effect of silymarin in experimental animal model of chronic inflammation. African J Pharm Pharmacol. 2009;3(5):242-247.
- Lalrinzuali K, Vabeiryureilai M, Jagetia GC. Investigation of the Anti-Inflammatory and Analgesic Activities of Ethanol Extract of Stem Bark of Sonapatha Oroxylum indicum In Vivo. Int J Inflam. 2016;2016:1-8.
- Mbiantcha M, Almas J, Shabana SU, Nida D, Aisha F. Anti-arthritic property of crude extracts of Piptadeniastrum africanum ( Mimosaceae ) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complement Altern Med. 2017;17(111):1-16.
- Bauerova K. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis Role of Reactive Oxygen and Nitrogen Species in Etiopathogenesis of Rheumatoid Arthritis. Gen Physiol Biophys. 2014;18:15-20.
- Cascão R, Vidal B, Raquel H, et al. Potent Anti-Inflammatory and Antiproliferative Effects of Gambogic Acid in a Rat Model of Antigen-Induced Arthritis. Mediators Inflamm. 2014;2014:1-7.
- Kshirsagar AD, Panchal P V., Harle UN, Nanda RK, Shaikh HM. Anti-inflammatory and antiarthritic activity of anthraquinone derivatives in rodents. Int J Inflam. 2014;2014:1-12.
- Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849-860.
