pp. 17-27
Apurva Agarwal, Gaurav Sharma and Nakuleshwar Dut Jasuja
Department of Applied and Biosciences, Suresh Gyan Vihar University, Jaipur
Corresponding Author: agarwalapurva00@gmail.com
ABSTRACT
Tuberculosis (TB) is one of the major causes of death in the world which is caused by Mycobacterium tuberculosis. The rate of mortality is high if it is conjugated with HIV. The multiple drug resistant strains make it difficult to diagnose and treat the disease. It can be easily spread around the world so there is a need to evolve new diagnostic methods that are rapid, cost effective and easy to use. Many methods have been used since years for diagnosis of disease. Some of the conventional methods are smear microscopy, chest radiography, cultivation of mycobacterium. But, in recent years many new molecular and advanced techniques have been used that are more specific and sensitive. These techniques are less time consuming and gives very few false positive results thereby, making diagnosis cost effective. In this article, some of the conventional and new advanced molecular diagnosis methods have been reviewed and there sensitivity and specificity has been taken into consideration.
Keywords: Diagnosis, tuberculosis, sensitivity.
INTRODUCTION:
Tuberculosis is the leading cause of death in the world which is caused by the infectious bacteria Mycobacterium tuberculosis. It mainly affects lungs but can affect other parts also like spine, kidney, brain, heart. It is ranked along with HIV in leading cause of deaths. Worldwide, 9.6 million people are estimated to have fallen ill with TB in 2014: 5.4 million men, 3.2 million women and 1.0 million children.
The person infected with HIV has high risk of TB. The emergence of drug resistant species has made it more difficult to diagnose and cure TB. Even today in India, two deaths occur every three minutes from TB (WHO Report, 2015). One woman on every 2.4 men is diagnosed with tuberculosis in India. India carries the burden of 30% tuberculosis population of the world (McArthur et al., 2016). The prevalence of Diabetes Mellitus in tuberculosis patients is high in South Eastern Amhara region (Workneh et al., 2016). Currently, more than 50 diagnostic methods are in development stage for the diagnosis of tuberculosis (Lienhardt et al., 2016). General Characteristics of mycobacterium tuberculosis: Mycobacterium tuberculosis is a large rod shaped non motile bacterium which is distantly related to the Actinomycetes. The length of these rods is 2-4 micrometers and width is 0.2-0.5 um. It is obligate aerobic in nature. For this reason, uberculosis is mainly found in lungs lobes where full aeration is provided. The bacterium has a slow generation time of around 15-20 hours. It cannot be classified as either gram positive or gram negative as it does not show any of the characteristics .But it contain murein in the cell wall so stains very weakly or not all with gram staining (cells referred to as “ghosts”).These are stained by carbol fuchsin stain and are referred to as acid fast bacilli.
Cell Wall Structure
The cell wall structure of Mycobacterium tuberculosis is a major determinant of virulence for the bacterium. The cell wall complex contains peptidoglycan. More than 60% of the mycobacterial cell wall is made up of lipid. The lipid portion of cell wall comprises of three major components, wax-D, mycolic acids and cord factors.
Mycolic acids are alpha-branched lipids that are present in cell walls of Mycobacterium. They make up 50% of the dry weight of the mycobacterial cell envelope is made by the mycolic acids. Mycolic Acids plays a significant role in virulence of mycobacterium. They prevent attack of the mycobacterium by cationic proteins, lysozyme, and oxygen radicals in the phagocytic granule. Cord factor is an inhibitor of PMN migration. It is toxic to mammals and roduced in large amount in virulent strains of Mycobacterium tuberculosis. Wax-D is the major component of Freund’s complete adjuvant (CFA).
The high concentration of lipids in the cell wall provides specialised and unique properties to bacterium. Some of them are;
• High concentration leads to provide resistance to many antibiotics.
• It leads to complement deposition which provides resistance to osmotic lysis.
• Provides survival in macrophages.
• provides resistance against acidic and alkaline compounds.
DIAGNOSIS OF TUBERCULOSIS:
The rapid and early diagnosis of disease is required to prevent the further infection and spread of disease.
Smear microscopy:
In most of the high burden resource poor countries, this method is used in which acid fast bacilli are detected in smear using a light microscope. The smears are stained with Ziehl-Neelsen (ZN) stain. But this technique is not so reliable. So in this regard fluorescence microscopy can be used against conventional light microscopy. The sensitivity was 52 to 97% as compared to light microscope with sensitivity of 32 to 94% (Parsons et al., 2011). One of the major advantage of fluorescence microscopy over ZN stain is that it is user friendly and the results are unaffected by HIV status of patient (Ndugga et al., 2003).
Sputum culture:
It is the most common method of diagnosis. In this method the sputum is cultured and then the growth of bacteria is observed. But it takes long time of around 2-4 weeks. Sputum Induction is a new approach which has a high yield in pulmonary tuberculosis. Most of the patients in pleural tuberculosis do not produce spontaneous sputum so this method can be used for effective diagnosis (Conde et al., 2003). Sputum induction gives best results when used with smear microscopy and can detect the patients who have negative spontaneous smear microscopy. (Hepple et al., 2012).
According to a study by Won-Jung Koh et al. a new approach was found which can minimize the time for tuberculosis detection on culture and drug susceptibility testing (DST). This is known as MGIT 960 method which decreases the turnaround time of DST to 27 days as compared to absolute concentration method which takes around 70 days (Koh et al., 2012). According to a study it was suggested that, with respect to the cost effectiveness in India, MGIT culture is more
preferred over serological tests as it gives less false positive results (Dowdy et al., 2011). Sputum induction can be easily and safely performed in children and infants and is more sensitive in both HIV infected and non- infected individuals (Hesseling et al., 2002) An assay known as microscopic observation brothdrug susceptibility assay (MODS) was used which has enhanced sensitivity of around 92% as well as uses less time for the detection of growth of mycobacterium in liquid culture (Caviedes et al., 2000).
Tuberculin skin test:
The Tuberculosis Skin Test (TST) has been in use for the diagnosis of tuberculosis infection since 1910. TST is a protein–purified derivate (PPD) method which results from a culture filtrate of tubercle bacilli containing over 200 antigens common both in bacilli Chalmette-Guerin vaccine (BCG) and in most non tuberculosis bacteria. Therefore, the specificity and accuracy of this test is low. Furthermore, it takes around 48-72 hours to read TST after initial administration. It can produce false results as error can be made in performing and reading of results.
A new advancement in this process has gain importance. The MPB64 skin patch test discriminates between active tuberculosis and PPD positive healthy controls and shows very high sensitivity of 98% with specificity 99% when tested in Japan. In Manila, Philippines it showed 100%specificity and 88%sensitivity (Drobniweski et al., 2003)
Detection of Lipoarabinomannan in Urine for Diagnosis of HIV-Associated TB:
Lipoarabinomannan (LAM) is a heat-stable glycolipid constituent in the cell wall of Mycobacterium tuberculosis. When mycobacterium releases this antigen it enters the circulation and then gets filtered in renal tubes, so easily detectable in urine of patients. It can be detected by simple sandwich ELISA using polyclonal antibodies. It could be used in cases where patient is infected with HIV and it can prove a powerful tool for diagnosis of tuberculosis in HIV patients (Achkar et al., 2011).
Fluorescence-activated cell sorting:
This technique can be used in suspects with negative AFB sputum smears. In this method BAL cells or sputum cells are immune phenotyped. But the frequency of region of difference-1 M. Tuberculosis specific T cells is too low in the
sputum to be used as stimulants for flow cytometry cultures and other immune based assays (Lange and Mori, 2010).
Polymerase Chain Reaction (PCR):
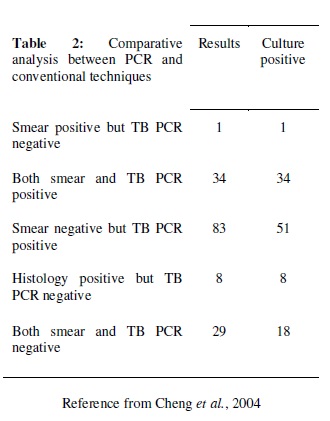
It was the widely used technique for the detection of tuberculosis. It is a rapid method for the diagnosis of disease. The technique is more sensitive than the other previously used methods. Out of the 144 patients that were tested it gave 80% positive result but smear examination gave only 25% accuracy (Cheng et al., 2004). In this approach, an experiment was carried out with PCR which was specific for devR and IS 6110 sequences. The sensitivity was 87.5% for the combined PCR results (Chakravorty et al., 2005).
The problem arises when the extrapulmonary tuberculosis is to be detected as it contains many inhibitors which interfere in amplification. So, proper treatment prior to reaction is necessary. So Cobas Amplicor MTB assay was used which eliminated all unwanted inhibitors (Bouakline et al., 2003).
The PCR was used to detect mycobacteria in urine sample using two methods- Sechi method and Githui method. But these methods were not that much sensitive that can be used for routine clinical diagnosis. The sensitivity of Sechi method was 28.6% and that of Githui method was 55.6% (Kafwabulula et al., 2002).
The PCR was conjugated with smear microscopy to diagnose the disease in smear-negative results. This proved better technique by detecting two targets devR and IS6110 sequences in the sample. These can prove a cost effective process for rapid diagnosis of smear-negative tuberculosis (Haldar et al., 2007). PCR requires proper treatment of sample prior reaction. So a new battery operated bead beating system is developed which can mechanically lyse the pathogen. This device is known as OmniLyse device developed by Claremont BioSolutions. It takes less than 10 minutes for the lysis of the pathogen. It improves the safety of lysis of mycobacteria before molecular diagnosis (Ferguson et al., 2016).
Recently, a new technique of end point detection has been used which used calcein for the visual detection of reaction. It binds to the manganese ions and produces fluorescence by quenching effect. This made ir simpleand cost effective that can be used in resource limited developing countries Park et al., 2003.
Phenotypic detection:
It is a method in which lux gene is incorporated in the phage which codes for lucifera phage allows the detection of mycobacteriophage by emitting light and thud allows the indirect detection of viable Mycobacterium tuberculosis. But this is quite expensive (Drobniewski 2003).
Molecular Typing Techniques:
Restriction fragment Length Polymorphism (RFLP) is the most commonly used technique based on insertion sequence IS6110. It has been used to study molecular evolution of strains, to identify laboratory cross-contaminations, for monitoring transmissions. (Palomino JC, 2005).
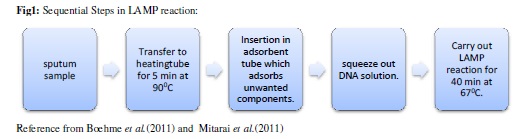
Loop mediated isothermal amplification (LAMP):
It is the reliable, cheap and effective technique for the diagnosis of tuberculosis. amplification technique which uses a
temperature and a single enzyme for the detection. It can amplify the DNA even if it is prese small quantity. (Tomita et al., results in 1 hour only. It uses Bst polymerase has polymerase activity as well as strand displacement activity. It has two inner primers and BIP) and two outer primers recognise six distinct regions in the target DNA.
This reaction causes the production of insoluble magnesium pyrophosphate during amplifica which appears as turbidity (Mori and Notomi, 2009).
The primer designing is very simple as it is done by primer design support software (http://primerexplorer.jp/e/). It automatically designs candid primer sets specific to target sequence. (Notomi et al., 2015).
The sensitivity of LAMP was increased by using the repetitive Insertion Sequence IS6110 as a target gene. This assay has 50 times more sensitivity then the gyrB as a target gene (Aryan et al., 2010). The combination of smear and LAMP is very sensitive and can be used as in-rule test combination (George et al., 2011).
LAMP has shown 100% sensitivity for culture positive and sputum positive results (Pandey et al., 2008). The PURE-LAMP has very high sensitivity and specificity which makes it economic, cost effective and rapid method for the diagnosis of tuberculosis (Ou et al., 2014). LAMP can be used for the RNA as well. Reverse transcriptase is added to the buffer and then the reaction is carried out (RT-LAMP). (Mori and Notomi, 2010).
The LAMP can be used for various applications like genetic testing, point of care testing, rapid testing of food products. But the major disadvantage is the risk of production of secondary products may be due to the high efficiency of the reaction. So it is recommended to prevent unwanted manipulations in master mix and reaction tubes and detection should be done carefully and separately. (Aryan et al., 2010).
In recent years, a new advancement in this technique is use of capillary tubes to run the reaction known as Microfluidic LAMP. The glass capillaries are placed in between the glass slides and the sample is injected in the capillaries. (Raftai and Gill, 2015).
Mycobacterium tuberculosis resistance to rifampicin (MTB-RIF) Assay:
In recent years, the direct nucleic acid amplification techniques have become a potential tool for the diagnosis of disease. In this approach, new technique in this field is evaluated which is realtime automated integrated system, the GeneXpert system using the Xpert MTB/RIF assay (GX).In this technique the sample is firstly treated with NaOH and isopropanol to make it inactive and then subjected to MTB/RIF cartridge and put in Xpert instrument. It leads to the DNA extraction and then amplification of 192-bp segment of the rpoB gene. The detection is done by the hybridisation of amplified product with the overlapping probes which consist of core regions of rpoB gene. GX has shown very high sensitivity for the detection of mutations and rifampicin resistance with 100% specificity (Moure et al., 2011).
The microscopic observation drug susceptibility (MODS) is a faster method which provides Drug Suseptibility Testing (DST). This technique provides diagnosis as well as detects resistance against rifampicin and isoniazid simultaneously (Kirwan et al., 2016).
In a study by Surendra Sharma et al. it was observed that the Xpert assay detected 71% of the ‘‘confirmed TB’’ cases where culture and response to anti-TB treatment were positive (Sharma et al., 2014). The pooled specificity of Xpert was 98% (Walusimbi et al., 2013).
The sensitivity for isoniazid resistance improves to 90% with the advanced MTB-DRplus version of the assay (Ling et al., 2008).
The comparison was made between two real time PCR based kits that are Cobas TaqMan MTB and Xpert MTB/RIF assay kit. The sensitivity of Cobas TaqMan was less as compared to Xpert MTB/RIF assay kit. The Xpert MTB/RIF was 100% sensitive as compared to Cobas TaqMan MTB which was 98% sensitive (Causse et al., 2011).
The high cost of Xpert and lack of impact on TB morbidity/mortality is a major issue (Naidoo et al., 2016). But the sensitivity and specificity of the test make it cost effective as there is no need to do multiple tests for diagnosis and there is reduced number of false positive results (Vassall et al., 2011).
Interferon- release assay (IGRA):
IGRAs are the alternatives of tuberculin skin tests and more sensitive and less time consuming (Santin et al., 2012). These are blood tests which measure ex vivo release of interferon-γ by T lymphocytes which are stimulated by antigens specific for Mycobacterium tuberculosis. These antigens are not found on BCG or most non tuberculosis
mycobacterium so these assays are more specific tests (Starke et al., 2014). Interferon γ release assays are useful for children diagnosis as ii indicates recent exposure which can later form active tuberculosis (Rhwald et al., 2012).
Early Secretory Antigen Target 6 (ESAT-6) and Culture Filtrate Protein 10 (CFP-10) are the antigens that are highly specific for active tuberculosis and can be used as a diagnostic reagent (Pinxteren et al., 2000). The Quantiferon- TB Gold test was conducted in which the whole blood stimulated with either of the M. tuberculosis–specific antigens Early Secretory Antigen Target 6 (ESAT-6) or Culture Filtrate Protein 10 (CFP-10) was taken. The result was considered positive when the amount of interferon- in the sample wells is greater than or equal to 0.35 IU/ml. It was shown that the test has around 89% sensitivity. But it is not yet used in routine clinical laboratories (Ferrara et al., 2005). These tests can prove beneficial for the diagnosis of active pulmonary tuberculosis where there is low prevalence of latent tuberculosis (Kang et al., 2007).
In a study by Nathan Shaviya et al. it was suggested that the IFN- levels and the ratios of IFN- to IL-10 and IFN- to adiponectin are associated with low body weight, body mass index, hip girth, diastolic pressure and plateletcrit suggesting mild-inflammation during early stage infection using newly infected TB patients. (Shaviya et al., 2015).
A new version in this field is T-SPOT assay which is enzyme linked immunosorbent spot (ELISPOT) assay. In this method the peripheral blood mononuclear cells are incubated with two antigens and then the results are detected as the number of interferon- producing T-cells that produces spot. If the number of spots exceeds the threshold, i.e. usually 8, after subtracting from the number of spots in negative control then the sample is considered positive.
These tests are costly then the other tests but economical as they generate very few false positive results and requires only a single visit of the individual. (Starke et al., 2014).
The most recent approach in this direction is the use of electrochemical immunosensor which is nanoparticle based. In this technique, the interferon ᵧ is captured by the magnetic nanoparticles and labelled with gold nanoparticles. It is then subjected to another anti interferon γ antibody and multiple cadmium sulfide nanoparticles form a complex. Then the electrochemical signals are recorded. It takes only around 1 hour as compared to ELISA which takes around double time. (Wang et al., 2016)
CONCLUSION:
In recent years, the diagnostic techniques have been advanced and became more reliable. The new approaches are used for making diagnosis more sensitive, economic and safe. But most of the advanced techniques are confined to the developed countries and are still not prevalent in developing countries. There is a need to make them available to the whole world so that this disease can be diagnosed at early stage and can be declined from the world. Tuberculosis can be eradicated by mutual efforts, proper planning, effective diagnosis, proper funding and continuous efforts in the field of research and development.
REFERENCES:
1. Adele Rafati & Pooria Gill. Microfluidic method for rapid turbidimetric detection of the DNA of Mycobacterium tuberculosis using loopmediated isothermal amplification in capillary tubes. Microchim Acta; 182:523–530 (2015).
2. Anna Vassall, Sanne van Kampen, Hojoon Sohn, Joy S. Michael, K. R. John, Saskia den Boon, J. Lucian Davis, Andrew Whitelaw, Mark P. Nicol, Maria Tarcela Gler, Anar Khaliqov, Carlos Zamudio, Mark D. Perkins, Catharina C. Boehme, Frank Cobelens. Rapid Diagnosis of Tuberculosis with the Xpert MTB/RIF Assay in High Burden Countries: A Cost Effectiveness Analysis. PLoS Med; 8.11: e1001120 (2011).
3. Basu Dev Pandey, Ajay Poudel, Tomoko Yoda, Aki Tamaru, Naozumi Oda, Yukari Fukushima, Binod Lekhak, Basista Risal, Bishnu Acharya, Bishwa Sapkota, Chie Nakajima, Tooru Taniguchi, Benjawan Phetsuksiri and Yasuhiko Suzuki. Development of an in-house loop-mediated isothermal amplification (LAMP) assay for detection of Mycobacterium tuberculosis and evaluation in sputum samples of Nepalese patients. Journal of Medical Microbiology; 57:439–443 (2008).
4. C. Hesseling, H. S. Schaaf, R. P. Gie, J. R. Starke, N. Beyers. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis.International Journal of Tuberculosis and Lung Diseases; 6.12:1038-1045 (2002).
5. Catharina C Boehme, Mark P Nicol, Pamela Nabeta, Joy S Michael, Eduardo Gotuzzo, RasimTahirli, Ma Tarcela Gler, Robert Blakemore, William Worodria, Christen Gray, Laurence Huang, Tatiana Caceres, Rafail Mehdiyev, Lawrence Raymond, Andrew Whitelaw, Kalaiselvan Sagadevan, Heather Alexander, Heidi Albert, Frank Cobelens, Helen Cox, David Alland, Mark D Perkins. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet; 377:1495–1505 (2011).
6. Catharina C. Boehme, Pamela Nabeta, German Henostroza, Rubhana Raqib, Zeaur Rahim, Martina Gerhardt, Erica Sanga, Michael Hoelscher, Tsugunori Notomi, Tetsu Hase, Mark D. Perkins. Operational feasibility of Using Loop-medaited isothermal amplification for the diagnosis of pulmonary tuberculosis in Microscopy Centres in Developing Countries. Journal of Clinical Microbiology; 45.6:1936-1940 (2007).
7. Christoph Lange and Toru Mori. Advances in the diagnosis of tuberculosis. Respirology ; 15:220–240 (2010).
8. D.I. Ling, A.A. Zwerling and M. Pai. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. European Respiratory Journal; 32.5: 1165–1174 (2008).
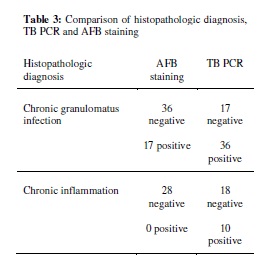
9. Do Youn Park, MD; Jee Yeon Kim, MD; Kyung Un Choi, MD; Jin Sook Lee, MD; Chang Hun Lee, MD; Mee Young Sol, MD; Kang Suek Suh, MD. Comparison of Polymerase Chain Reaction with Histopathologic Features for Diagnosis of Tuberculosis in Formalin-Fixed, Paraffin-Embedded Histologic Specimens. Arch Pathol Lab Med; 127:326–330 (2003).
10. Dowdy DW, Steingart KR, Pai M. Serological Testing Versus Other Strategies for Diagnosis of Active Tuberculosis in India: A Cost Effectiveness Analysis. PLoS Med; 8.8: e1001074 (2011).
11. Ehsan Aryan, Manoochehr Makvandi, Ahmad Farajzadeh, Kris Huygen, Pablo, Bifani, Seyed-Latif Mousavi, Abolfazl Fateh, Abbass Jeoldar, Mohammad-Mehdi Gouya, Marta Romano. A novel and more sensitive loopmediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiolgical Research; 165:211-220 (2010).
12. F A Drobniewski, m Caws, A Gibson, D Young. Modern Laboratory diagnosis of tuberculosis. Lancet Infectious diseases; 3:141-147 (2003).
13. Ferguson TM, Weigel KM, Lakey Becker A, Ontengco D, Narita M, Tolstorukov I, Doebler R, Cangelosi GA, Niemz A. Pilot study of a rapid and minimally instrumented sputum sample preparation method for molecular diagnosis of tuberculosis. Sci. Rep.; 6:19541 (2016).
14. George G, Mony P, Kenneth J. Comparison of the Efficacies of Loop-Mediated Isothermal Amplification, Fluorescence Smear Microscopy and Culture for the Diagnosis of Tuberculosis. PLoS ONE; 6.6:e21007 (2011).
15. Giovanni Ferrara, Monica Losi, Marisa Meacci, Barbara Meccugni, Roberto Piro, Pietro Roversi, Barbara Maria Bergamini, Roberto D’Amico, Patrizia Marchegiano, Fabio Rumpianesi, Leonardo M. Fabbri, and Luca Richeldi. Routine Hospital Use of a New Commercial Whole Blood Interferon-Assay for the Diagnosis of Tuberculosis Infection. American Journal of Respiratory and Critical Care Medicine; 172:631-635 (2005).
16. Issar Smith. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clinical Microbiology Reviews; 16.3:463–496 (2003).
17. J.C. Palomino. Nonconventional and new methods in the diagnosis of tuberculosis: feasibility and applicability in the field. European respiratory Journal; 26:339-350 (2005).
18. Jacqueline M. Achkar, Stephen D. Lawn, Mahomed-Yunus S. Moosa, Colleen A. Wright, and Victoria O. Kasprowicz. Adjunctive Tests for Diagnosis of Tuberculosis: Serology, ELISPOT for Site-Specific Lymphocytes, Urinary Lipoarabinomannan String Test, and Fine Needle Aspiration. The Journal of Infectious Diseases; 204:1130-1141 (2011).
19. Jeffrey R. Starke, FAAP and Committee on Infectious Diseases. Interferon-γ Release Assays for Diagnosis of Tuberculosis Infection and Disease in Children.American Academy of Pediatrics; 134.6:e1763-1773 (2014).
20. Kirwan DE, Ugarte-Gil C, Gilman RH, Caviedes L, Rizvi H, Ticona E, Chavez, G, Cabrera JL, Matos ED, Evans CA, Moore DAJ, Friedland JS, the Lymph Node TB Working Group, Peru. Microscopic observation drug susceptibility assay for rapid diagnosis of lymph node tuberculosis and detection of drug resistance. Journal of Clinical Microbiolgy; 54:185–189 (2016).
21. L. E. A. Kivihya-Ndugga, M. R. A. van Cleeff, W. A. Githui, L. W. Nganga, D. K. Kibuga, J. A. Odhiambo, Paul R. Klatser. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource-poor urban setting. International Journal of Tuberculosis and Lung Disease; 7.12:1163–1171 (2003).
22. Laurens A. H. Van Pinxteren, Pernille Ravn, Else Marie Agger, John Pollock, and Peter Andersen. Diagnosis of Tuberculosis Based on the Two Specific Antigens ESAT-6 and CFP10. Clinical and Diagnostic Laboratory Immunology; 7.2:155-160 (2000).
23. Christian Lienhardt, Knut Lönnroth, Dick Menzies, Manica Balasegaram, Jeremiah Chakaya, Frank Cobelens, Jennifer Cohn, Claudia M. Denkinger, Thomas G. Evans, Gunilla Källenius, Gilla Kaplan, Ajay M. V. Kumar, Line Matthiessen, Charles S. Mgone, Valerie Mizrahi, Ya-diul Mukadi, Viet Nhung Nguyen, Anders Nordström, Christine F. Sizemore, Melvin Spigelman, S. Bertel Squire, Soumya Swaminathan, Paul D. Van Helden, Alimuddin Zumla, Karin Weyer, Diana Weil, Mario Raviglione. Translational Research for Tuberculosis Elimination: Priorities, Challenges, and Actions. PLoS Med; 13.3:e1001965 (2016).
24. Linda M. Parsons, A´ kos Somosko¨vi, Cristina Gutierrez, Evan Lee, C. N. Paramasivan, Alash’le Abimiku, Steven Spector, Giorgio Roscigno and John Nkengasong. Laboratory Diagnosis of Tuberculosis in Resource-Poor Countries: Challenges and Opportunities. Clinical Microbiology Reviews; 24.2:314-350 (2011).
25. Luz Caviedes, Tien-Shun Lee, Robert H. Gilman, Patricia Sheen, Emily Spellman, Ellen H. Lee, Douglas E. Berg, Sonia Montenegro-James And The Tuberculosis Working Group In Peru. Rapid, Efficient Detection and Drug Susceptibility Testing Of Mycobacterium Tuberculosis In Sputum By Microscopic Observation Of Broth Cultures. Journal of Clinical Microbiology; 38.3:1203-1208 (2000).
26. M. Kafwabulula, K. Ahmed, T. Nagatake, J. Gotoh, S. Mitarai, K. Oizumi, A. Zumla. Evaluation of PCR-based methods for the diagnosis of tuberculosis by identification of mycobacterial DNA in urine samples. International Journal of Tuberculosis and Lung Disease; 6.8:732-737 (2002).
27. Manuel Causse, Pilar Ruiz, Juan Bautista Gutie´rrez-Aroca, and Manuel Casal. Comparison of Two Molecular Methods for Rapid Diagnosis of Extrapulmonary Tuberculosis. Journal of Clinical Microbiology; 49.8:3065-3067 (2011).
28. Marcus B. Conde, Angela Chindamo Loivos, Valeria M. Rezende, Sergio L. M. Soares, Fernanda C. Q. Mello, Arthur L. Reingold, Charles L. Daley, and Afranio L. Kritski. Yield of Sputum Induction in the Diagnosis of Pleural Tuberculosis. American Journal Of Respiratory And Critical Care Medicine; 167:723-725 (2003).
29. McArthur E, Bali S, Khan AA. Sociocultural and knowledge-based barriers to tuberculosis diagnosis for women in Bhopal, India. Indian J Community Med; 41:62-64 (2016).
30. Morten Ruhwald, Martine G Aabye & Pernille Ravn. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Review of Molecular Diagnostics; 12:2:175-187 (2012).
31. Pren Naidoo, Rory Dunbar, Carl Lombard, Elizabeth du Toit, Judy Caldwell, Anne Detjen, S. Bertel Squire, Donald A. Enarson, Nulda Beyers. Comparing Tuberculosis Diagnostic Yield in Smear/Culture and Xpert MTB/RIF-Based Algorithms Using a Non- Randomised Stepped-Wedge Design. PLoS ONE; 11.3: e0150487 (2016).
32. Nathan Shaviya, Valentine Budambula, Mark K. Webale, and Tom Were. Circulating Interferon-Gamma Levels Are Associated with Low Body Weight in Newly Diagnosed Kenyan Non-Substance Using Tuberculosis Individuals. Interdisciplinary Perspectives on Infectious Diseases; 2016:1-9 (2015).
33. Norihiro Tomita, Yasuyoshi Mori, Hidetoshi Kanda and Tsugunori Notomi. Loop-mediated isothermal amplification of gene sequences and simple visual detection of products. Nature protocols; 3.5:877-882 (2008).
34. P. Hepple, N. Ford, R. McNerney. Microscopy compared to culture for the diagnosis of tuberculosis in induced sputum samples: a systematic review. The International Journal of Tuberculosis and Lung Disease; 16.5:579–588 (2012).
35. Raquel Moure, Laura Munoz, Miriam Torres, Miguel Santin, Rogelio Martín and Fernando Alcaide. Rapid Detection of Mycobacterium tuberculosis Complex and Rifampin Resistance in Smear-Negative Clinical Samples by Use of an Integrated Real-Time PCR Method. Journal of Clinical Microbiology; 49.3:1137–1139 (2011).
36. S. Honore´-Bouakline, J. P. Vincensini, V. Giacuzzo, P. H. Lagrange, and J. L. Herrmann. Rapid Diagnosis of Extrapulmonary Tuberculosis by PCR: Impact of Sample Preparation and DNA Extraction. Journal of Clinical Microbiology; 41.6:2323-2329 (2003).
37. S. Mitarai, M. Okumura, E. Toyota, T. Yoshiyama, A. Aono, A. Sejimo, Y. Azuma, K. Sugahara, T. Nagasawa, N. Nagayama, A. Yamane, R. Yano, H. Kokuto, K. Morimoto, M. Ueyama, M. Kubota, R. Yi, H. Ogata, S.Kudoh, T. Mori.
Evaluation of a simple loop-mediated isothermal amplification test kit for the diagnosis of tuberculosis. International Journal of Tuberculosis and Lung Diseases; 15.9:1211-1217 (2011).
38. Sagarika Haldar, Soumitesh Chakravorty, Manpreet Bhalla, Shyamasree De Majumdar and Jaya Sivaswami Tyagi. Simplified detection of Mycobacterium tuberculosis in sputum using smear microscopy and PCR with molecular beacons. Journal of Medical Microbiology: 56:1356–1362 (2007).
39. Santin M, Mun˜oz L, Rigau D. Interferon-ᵧ Release Assays for the Diagnosis of Tuberculosis and Tuberculosis Infection in HIV-Infected Adults: A Systematic Review and Meta-Analysis. PLoS ONE; 7(3): e32482 (2012).
40. Simon Walusimbi, Freddie Bwanga, Ayesha DeCosta, Melles Hail,Moses Joloba and Sven Hoff ner. Meta-analysis to compare the accuracy of GeneXpert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC infectious diseases; 13:507 (2013).
41. Soumitrsh Chakravorty, Manas Kamal Sen, Jaya Sivaswami Tyagi. Diagnosis of Extrapulmonary Tuberculosis by Smear, culture and PCR using Universal Sample Processing Technology. Journal of Clinical Microbiology; 43.9:4357-4362 (2005).
42. Surendra K. Sharma, Mikashmi Kohli, Jigyasa Chaubey, Raj Narayan Yadav, Rohini Sharma, Binit Kumar Singh,Vishnubhatla Sreenivas, Abhishek Sharma, Rohit Bhatia, Deepali Jain, V. Seenu, Anita Dhar and Manish Soneja. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care centre in India. European Respiratory Journal;In press (2014).
43. Tsugunori Notomi, Yasuyoshi Mori, Norihiro Tomita, and Hidetoshi Kanda. Loopmediated isothermal amplification (LAMP): principle, features and future prospects. Journal of Microbiology; 53.1:1–5 (2015).
44. VCC Cheng, WC Yam, IFN Hung, PCY Woo, SKP Lau, bSF Tang, KY Yuen. Clinical evaluation of the polymerase chain reaction for the rapid diagnosis of tuberculosis. Journal of Clinical Pathology; 57:281-285 (2004).
45. Won-Jung Koh, Yousang Ko, Chang-Ki Kim, Kyung Sun Park and Nam Yong Lee. Rapid Diagnosis of Tuberculosis and Multidrug Resistance Using a MGIT 960 System. Annals of Laboratory Medicine; 32:264-269 (2012).
46. Workneh MH, Bjune GA, Yimer SA. Prevalence and Associated Factors of Diabetes Mellitus among Tuberculosis Patients in South Eastern Amhara Region, Ethiopia: A Cross Sectional Study. PLoS ONE; 11.1:e0147621. (2016).
47. Xichao Ou, Qiang Li, Hui Xia., Yu Pang, Shengfen Wang, Bing Zhao, Yuanyuan Song, Yang Zhou, Yang Zheng, Zhijian Zhang, Zhiying Zhang, Junchen Li, Haiyan Dong, Jack Zhang, Kai Man Kam, Junying Chi, Shitong Huan, Daniel P. Chin, Yanlin Zhao. Diagnostic Accuracy of the PURELAMP Test for Pulmonary Tuberculosis at the County-Level Laboratory in China. PLoS ONE; 9.5: e94544 (2014).
48. Yasuyoshi Mori, Tsugunori Notomi. Loopmediated isothermal amplification (LAMP): a rapid, accurate and cost-effective diagnostic method for infectious disease. Japanese Society of Chemotherapy and the Japanese Association for Infectious Disease; 15:62-69 (2009).
49. Young Ae Kang, Hye Won Lee, Seung Sik Hwang, Sang-Won Um, Sung Koo Han, Young- Soo Shim and Jae-Joon Yim. Usefulness of Whole- Blood Interferon-ᵧAssay and Interferon- ᵧ Enzyme- Linked Immunospot Assay in the Diagnosis of Active Pulmonary Tuberculosis. Chest; 132:959– 965 (2007).
50. Yun Wang, Gerald H. Mazurek and Evangelyn C. Alocilja. Measurement of Interferon Gamma Concentration Using an Electrochemical Immunosensor. J. Electrochem. Soc.; 163.5:B140- B145 (2016).
