Hanmant S. Mali1*, Safiya R. Shaikh2, Akshay R. Yadav3, Indrayani D. Raut4, Mangesh A. Bhutkar5, Shrinivas K. Mohite6, Chandrakant S. Magdum7
1,4,5Department of Pharmaceutics, Rajarambapu College of Pharmacy, Kasegaon, Dist-Sangli, Maharashtra, India-415404
2,3,6,7Department of Pharmaceutical Chemistry, Rajarambapu College of Pharmacy, Kasegaon, Dist-Sangli, Maharashtra, India-415404
ABSTRACT
SEDDS are used to poorly soluble, highly permeable, and low bioavailability compounds. In terms of orally administered drug solubility, the pharmaceutical industry faces a major challenge, as nearly 35-40% of newly launched drugs have low aqueous solubility, resulting in poor dissolution and low bioavailability. Salt forming, solid dispersion, and complex formation are some of the methods that can help with this. The Self-Emulsifying Drug Delivery System (SEDDS) is gaining popularity as a way to improve the solubility of lipophilic medications. After gentle agitation and dilution in aqueous media such as GI fluids. The current research provides an up-to-date summary of SEDDS advancements in terms of composition, evaluation, numerous dosage forms, and novel procedures for converting liquid SEDDS to solid, as well as a variety of applications.
Keywords: Self-Emulsifying Drug Delivery System, Aqueous solubility, Isotropic mixtures.
INTRODUCTION
Due to their lack of considerable solubility in water, it is believed that 40% of active compounds found by combinatorial screening procedures are challenging to synthesize1. Producing multiple salt forms is one of the conventional ways used to improve the solubility of a weakly water soluble molecule without compromising its biological activity. The use of a prodrug has also increased solubility. However, in the vast majority of situations, such efforts fail, and molecules must be abandoned during the development stage. It’s possible that a product will be released with inferior qualities, such as low bioavailability or incorrect dosing2. Surfactants, permeation enhancers, micronization, nanoparticles, solid dispersions, and other techniques have been used to overcome these sub-optimal features3. Most weakly water-soluble medicines have had their solubility and oral bioavailability increased by SEDDs4. Though emulsion systems have proven to be effective for encapsulating poorly water-soluble pharmaceuticals, they do have several drawbacks, such as stability, which might cause issues during commercial production5. On modest agitation, they immediately form fine oil-in-water emulsions, which are then diluted with aqueous solution (for example: gastrointestinal fluid)6 When the formulation forms transparent microemulsions with oil droplets ranging from 100 to 250 nm, SEDDs are referred to as self-microemulsifying drug delivery systems (SMEDDs)7. SMEDDs are principally useful for oral delivery of lipophilic and poorly water-soluble drugs. This is due to their ability to spontaneously emulsify at a given temperature ability to be sterilised by filtration, their solubilizing property and high physical stability8.
- Advantages
- Improved patient compliance.
- Reduced dosing frequency.
- Ability to deliver active biomolecules along with GIT-sensitive peptides to enzymatic hydrolysis.
- Unlike the other lipid-based drug delivery systems, no influence of lipid digestion process.
- When polymer is incorporated into SMEDDS composition it provides prolonged release of medicinal products.
- Disadvantages
- Lack of good in-vitro predicative assessment models.
- High surfactant concentrations (30-60%) irritate.
- Lack of good IVIVC correlation and appropriate animal model for in vivo study.
- Limitations of SMEDDS
(1) Encapsulation in soft gelatin capsules:
The majority of SMEDDS formulations are sold as soft gelatin capsules. Gelatin capsules, on the other hand, have a few disadvantages. These issues have prompted a market need for a soft gelatin capsule alternative36. Capsules made from HPMC are currently the preferred alternative to animal gelatin capsules. The HPMC capsule shell has been investigated as a possible option to encapsulating supersaturable SMEDDS formulations37.
(2) Storage and handling:
Handling, storage, and stability issues plague liquid SMEDDS. As a result, developing reliable SMEDDS appears to be a natural answer to these issues38.
(3) Limited targeting to lymphatic’s:
Targeting lymphatics has two major advantages over traditional portal blood absorption. For starters, transport via the intestinal lymph bypasses pre-systemic hepatic processing, increasing the concentration of orally delivered medicines in the systemic circulation. Second, drug distribution to lymphatic organs could be site-specific. For lymphatic transport, high triglyceride solubility and a high log P are necessary39-40
(4) Lack of good in vitro models:
Traditional dissolve procedures are ineffective because these formulations may be dependent on lipid decomposition in the gut prior to medication release. In order to replicate this, an in vitro model of the duodenum’s digestion processes was created41. Before the strength of this in vitro model can be assessed, it must be refined and validated38.
(5) Rational for Self-Micro Emulsifying Drug Delivery System
When delivered orally to the gastrointestinal tract, BCS class II or class IV substances are often dissolution rate limited. Currently, there is no one or simple answer to the problem. For this, a variety of formulation methodologies can be used. In certain circumstances, these tactics have shown to be effective. These solutions, however, have their own set of restrictions. Reduction of particle size may not be desirable in situations where very fine powders experience handling difficulty and poor wettability.
Table 1: Basic Difference and Similarities between SEDDS and SMEDDS9, 12
| SMEDDS | SEDDS |
| DIFFERENCES | |
| 1. SMEDDS are thermodynamically stable in Water or physiological fluid. | 3. SEDDS are not thermodynamically stable in water or physiological fluid. |
| 2. Concentration of oil <20%. | 4. Concentration of oil is 40-80% |
| 3. Optimization of SMEDDS require Pseudo ternary phase diagram. | 5. Optimization of SEDDS require ternary Phase diagram. |
| SIMILARITIES | |
| Form fine oil-in-water dispersion in contact with GIF | |
- Mechanism of self-emulsification
Emulsions are created by stirring two immiscible liquids, such as water and oil, with the addition of surfactants, which causes the surface area of the two phases to expand. Surface active agents generate a layer surrounding the internal phase of the emulsion droplet, which helps to stabilise it. The excess surface free energy in this scenario is determined by particle size and interfacial tension14. SMEDDS self-emulsification is linked to free energy. They generate thermodynamic spontaneous emulsification because they have a positive and very low free energy, or even a negative free energy. This could be due to the mild agitation during the procedure allowing water to penetrate the Liquid Crystalline (LC) phase. Water penetration comes to a halt at a particular point and forms droplets. This phase is suitable for the emulsions’ great stability. The three key parameters that influence SMEDDS are drug concentration, phase polarity, and drug solubility. The polarity of the lipid phase controls drug release from the micron emulsion. For SMEDDS, drugs that are poorly soluble and taken at greater doses are not recommended. The drug’s solubility is a crucial parameter for SMEDDS16. Certain combinations of pharmaceutical excipients are required for the process to work. The concentration of surfactant, as well as the temperature at which self-emulsification occurs, all depend on the surfactant pair and oil ratios. The discovery of specific combinations of excipients and the construction of a phase diagram that shows various concentrations are the first steps in the formulation process17.
- Appropriate drug candidates for SMEDDS
SMEDDs improve the pace and extent of absorption of lipophilic/hydrophobic medicines with dissolution-limited absorption.18 This could lead to repeatable time patterns. The SMEDDS technique, on the other hand, may be applied to all medications in the biopharmaceutical categorization system (BCS) 7. Table 2 highlights the numerous challenges that SMEDDs can tackle. Drug properties like aqueous solubility and/or log P may not be enough to determine the feasibility of a lipid-based formulation because they can’t accurately predict potential in vivo effects6.
- Formulation of SMEDDS
SMEDDS formulation containing following components:
- Oil phase
SMEDDS systems must be produced with non-toxic and safe components in order to be pharmaceutically acceptable. Natural oil and its derivatives are regarded environmentally safe. In model studies and for use in industrial goods, the generation of bicontinuous microemulsions with mineral oils has been extensively researched20. In general, the extension of a microemulsion region depends on the nature of the oil. This is because of differences in the penetration of oil into the surfactant layer21.
- Surfactants
Surfactants generate an interfacial coating and reduce interfacial tension to a low level, making the dispersion process easier. When choosing a surfactant, the HLB value and surfactant concentration must be taken into account22.
- Co-surfactants
Co-surfactants of a medium chain length (C3–C8) are often used23.
- Co-solvents
High surfactant concentrations (usually more than 30%) are required for optimal SMEDDS formation. During the oil phase, organic solvents allow considerable amounts of either the hydrophilic surfactant or the medication to be dissolved. Ethanol, butanol, and propylene glycol are examples, as are esters like ethyl propionate and tributyl citrate, and amides like 2-pyrolidine, caprolactum, and polyvinyl pyrollidine24-25.
- Method of Preparation26
- Phase Titration Method
The process of spontaneous emulsification (phase titration method) is used to make microemulsions, which can be represented using phase diagrams. Microemulsions are generated with diverse association structures depending on the chemical content and concentration of each component.
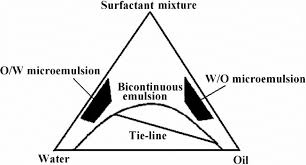
Fig-1: Phase Diagram
- Phase inversion Method
When the dispersed phase excess is added or when the temperature is changed, phase inversion of microemulsions occurs. Rapid physical changes, such as changes in particle size, occur during phase inversion,.
- Release mechanism:29-30
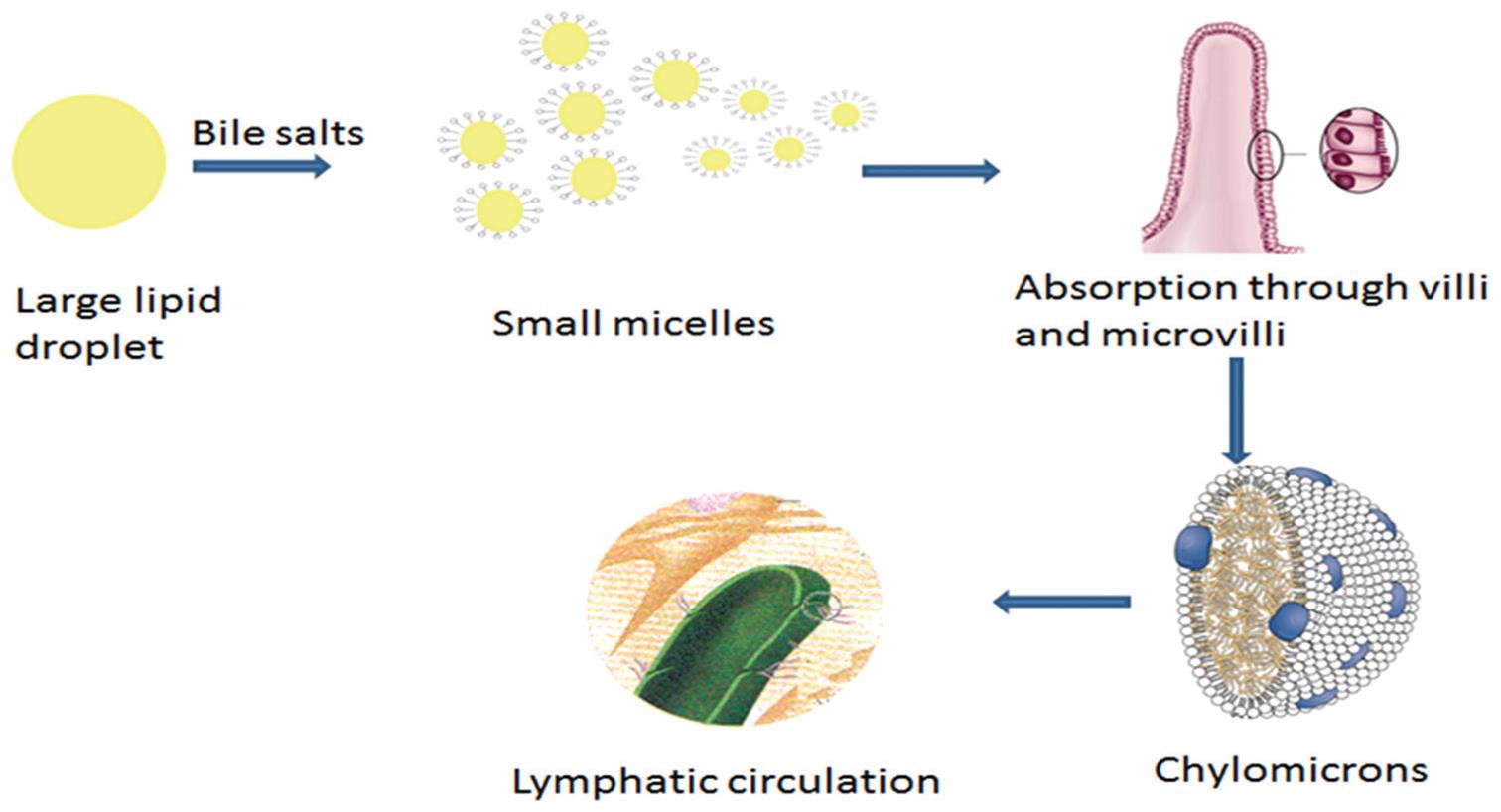
Fig-2: Absorption mechanism of lipid-based system i.e. SMEDDS
When triglyceride droplets reach the upper region of the small intestine, pancreatic lipase breaks them down into free fatty acids and 2-monoglycerides, the latter two of which aid digestion by acting as emulsifiers25.
- Characterizations of SMEDDS31-46
The various forms of characterization of SMEDDS are summarized below;
- Visual assessment
Visual inspection is the most common method of determining self-emulsification. This could reveal important details regarding the mixture’s self-emulsifying and micro-emulsifying properties, as well as the dispersion that results.
- Turbidity measurement
This evaluates self-emulsification efficiency by determining if the dispersion finds equilibrium quickly and in a repeatable time. Turbidity metres are used to make these measurements (Hach turbidity metre and the Orbeco-Helle turbidity meter).
- Droplet size
To assess the droplet size of an emulsion, microscopic methods, photon correlation spectroscopy, or a Coulter Nanosizer are commonly utilised.
- Determination of emulsification time
This method is used to estimate the amount of time required for emulsification. The effectiveness of emulsification of various surfactant and lipid compositions is measured in a nephelometer utilising a revolving paddle to induce emulsification.
- Particle size distribution
The particle size distribution of the microemulsion is measured using dynamic light scattering techniques. The velocity of Brownian diffusion and, as a result, the dispersed droplets are measured using the fluctuation in scattered light intensity. Cryogenic transmission electron microscopy can be used to confirm particle size distributions (cryo-TEM). The advantage of cryo-TEM is that it allows you to see the particle sizes and shapes32.
- Conductivity measurements
The point at which the aqueous process is introduced and the system transitions from receiving continuous oil to receiving continuous water will be determined by conductivity measurements. It also aids in the detection of percolation or phase inversion33.
- Refractive index and percent transmission
The correctness of the formulation is demonstrated by the refractive index and percentage transmittance. A refractometer is used to determine the SMEDDS refractive index, which is then compared to that of water. Transmittance is transparent in nature > 99 percent, according to this formula35-39.
- Zeta potential measurement
In triplicate samples, the zeta potential for microemulsion can be calculated using an effective Zetasizer40-44.
- Centrifugation
Centrifuged thaw cycles between 21°C and +25°C are performed, with storage for at least 48 hours at 3500 rpm for 30 minutes at each temperature. The formulations that do not show any phase separation will be used in the freeze-thaw stress test45-50.
- In vitro release study
The dialysis method, dissolution equipment II, and diffusion layer were used to examine SMEDDs formulation in vitro drug release. The drug release test was carried out in a 200 mL buffer solution with a pH of 6.8 and a modified diffusion layer. The beaker was continually swirled with a magnetic stirrer, and samples were removed at various time intervals and examined using a UV Spectrophotometer51-56.
CONCLUSION
Drug, lipid phase, emulsifier, and/or co-solvent mixes make up self-emulsifying drug delivery systems. SEDDS are a promising strategy for medications with low aqueous solubility, and as a result. The larger surface area of the finer droplets allows the drug to dissolve or penetrate into the surrounding medium. Solid SEDDS (tablets, capsules, beads, microspheres, etc.) are favoured over liquid SEDDS because they are easier to handle, transport, and have better stability.
REFERENCES
- Lipinski C. Drug-like properties and the causes of poor solubility and poor permeability. Journal of Pharmacological and Toxicological methods. 2000; 235: 249-44.
- Elaine M, Merisko L, Gary GL. Drug Nanoparticles: Formulating Poorly Water-Soluble Compounds, Toxicological Pathology. 2008; 36: 43.
- Pouton CW. Lipid formulations for oral administration of drugs: nonemulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. European Journal of Pharmaceutical Sciences. 2000; 11 (2): S93–S98.
- Gursoy N, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomedicine & Pharmacotherapy. 2004; 173: 182-58.
- Venkatesh G, Majid MI, Mansor SM, Nair NK, Croft SL, Navaratnam V. In vitro and in vivo evaluation of self-microemulsifying drug delivery system of buparvaquone. Drug Development and Industrial Pharmacy. 2010; 36(6): 735-45.
- Kohli K, Chopra S, Dhar D, and Arora S, Khar RK. Self emulsifying drug delivery systems: An approach to enhance oral bioavailability. Drug Discovery Today. 2010; 15(21-22): 958-65.
- Singh B, Bandopadhyay S, Kapil R and Singh R, Katare OP. Self-emulsifying drug delivery systems (SEDDS): Formulation develop-ment, characterization, and applications. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2009; 26(5): 427–521.
- Sarciaux JM, Acar L, Sado PA. Using microemulsion formulations for oral drug delivery of therapeutic peptides. International Journal of Pharmaceutics. 1995; 120: 127-36.
- A Review on self-micro-emulsifying drug delivery system. Pharma science monitor. 2013; 4(1), 3629-3630
- Dhadde G.S, Yadav J.P , Sapate R.B, Mali H.S, Raut I.D, In vitro Anthelmintic Activity of crude extract of flowers of Bougainvillea Spectabilis Wild against PheretimaPosthuma, International Journal of Pharmacy and Pharmaceutical Research, 2020; 17: 1-18.
- Tang et al. Self-Emulsifying Drug Delivery Systems: Strategy for Improving Oral Delivery of Poorly Soluble Drugs. Current Drug Therapy. 2008; 2: 85-93.
- Jessy, S and Joshi, V. Self-Micro Emulsifying Drug Delivery System (SMEDDS) For Improving Bioavailability of Hydrophobic Drugs And Its Potential To Give Sustain Release Dosage Form. Indian Journal of Pharmaceutical Education. 2005; 39(3); 130-135.
- Yadav A, Mohite S. Aquasomes as a Self Assembling Nanobiopharmaceutical Carrier System for Bio-Active Molecules. Research J. Topical and Cosmetic Sci. 2020; 11(2): 66-70.
- Chaudhary et al. Self-Emulsifying Drug Delivery System: A Novel Approach for Enhancement of Bioavaibility, Journal of Pharmaceutical Science and Bioscientific Research. 2011; 1(1), 31-36.
- Akanksha Jagdale, Dr. Sandeep Patil, Hanmant Mali, Snehal Chandanshive, In-vitroAntispasmodic Efficiency of Ethanolic Extract of Leaves of Sesbania Grandiflora, World Journal Of Pharmaceutical Research, 2020; 9(2): 915-921.
- Yadav A, Mohite S. Potential Role of Peptides for Development of Cosmeceutical skin Product. Research J. Topical and Cosmetic Sci. 2020; 11(2): 77-82.
- Laxmikant T Gadhe, Sameeran V Kapadi, Baburao Bachkar and Mayur Gandhi, Swati talele, Chaudhari G.N. Recent patent review on self-micro emulsifying drug delivery system. World Journal of Pharm. Res. 2015; 4 (3): 965-979.
- Yadav A, Mohite S. Applications of Nanotechnology in Cosmeceuticals. Research J. Topical and Cosmetic Sci. 2020; 11(2): 83-88.
- P. Wankhade, Nivedita. S. Kale and Tapar. K.K. Self-micro emulsifying nutraceutical and drug delivery systems. Int. J. Pharm. Sci and Nanotec. 2014; 7 (3): 2520-2528.
- Dhadde Gurunath S., Mali Hanmant S., SapateRohit B., VakhariyaRohan R., RautIndrayani D., NitalikarManoj M., Investigation of In-Vitro Anthalmintic Activity of Methanolic Extract of TylophoraIndica Leaves Against HaemonchusContortus, Journal of University of Shanghai for Science and Technology, 2021; 23(2): 37-42.
- Yadav A, Mohite S. Recent advances in protein and peptide drug delivery. J. Pharma. Dosage Forms and Tech. 2020; 12(3): 205-212.
- Barkat Ali Khan, Satar Bakhsh, Haroon Khan, Tariq Mahmood, Akhtar Rasul. Basics of Self micro emulsifying drug delivery system, J. Pharm. and Alt. Med. 2012; 1: 13-19.
- Honmane P, Yadav A, Singh S, Mohite S. 3D printing technology in pharmaceuticals and biomedical. World J Pharm Pharm Sci. 2020; 9(9): 598-609.
- O’Driscoll CM, Griffin BT. Biopharmaceutical challenges associated with drugs with low aqueous solubility – the potential impact of lipid-based formulations. Advanced Drug Delivery Review. 2008; 60: 617-24.
- Dhadde Gurunath S., Mali Hanmant S., Raut Indrayani D., Nitalikar Manoj M.,Bhutkar Mangesh M., A Review on Microsphere: Types, Method of Preparation, Characterization and Application, Asian Journal of Pharmacy and Technology. 2021; 11(2):149-155.
- Lin, S.L.; Menig, J. and Lachman, L. Interdependence of physiological surfactant and drug particle size on the dissolution behavior of water-insoluble drugs. J. Pharm. Sci.; 1968; 2143-2148.
- Salunkhe K, Yadav A. Recent Advances in Manufacturing Technologies and Future Prospects of Mouth Dissolving Tablets (FDTs). International Journal of Scientific Research in Chemistry. 2020; 5(2): 26-32.
- Shford, D. M. and Craig D. Q. M. The emulsification and solubilization properties of polyglycolysed oils in self-emulsifying formulations. J. Pharm. Pharmacol.; 2004; 56; 307-316.
- Suryawanshi V, Yadav A, Birajdar R. Optimization of Ayurvedic Herbal Medicine by Nanoformulation. Asian Journal of Research in Pharmaceutical Sciences. 2019; 9(1): 55-56.
- Azaz E, Donbrow M, Hamburger R. Incompatibility of non-ionic surfactants with oxidisable drugs. Pharm. 1973. J 211:15.
- Humberstone AJ, Charman WN. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv Drug Deliv Rev. 1997. 25:103–28.
- Shinde et al. Novel Approaches for Development and Characterization of SMEDDS: Review. International Journal of Current Pharmaceutical Research. 2013; 5(4): 5-12.
- Agrawal et al. A Review on Novel Therapeutic Strategies for the Enhancement of Solubility for Hydrophobic Drug Through Lipid and Surfactant Based Self Micro Emulsifying Drug Delivery System: A Novel Approach. American Journal of Drug Discovery & Development. 2013; 2(4), 143-183.
- Talegoanker, A. Azeem and F. J. Ahmad. A novel approach to enhance drug delivery: Microemulsion, Recent Patents on Drug del. & formulation, 2008, 2: 238-257.
- Pouton, C.W. et al. Self-emulsifying systems for oral delivery of drugs. International Symposium on Control Release Bioactive Materials pp. 1987; 113–114.
- Gursoy, N. et al. Excipient effects on in vitro cytotoxicity of a novel paclitaxel self-emulsifying drug delivery system. J. Pharm. Sci. 2003; 92:2411–2418. Palamakula A. Khan M.A. Evaluation of cytotoxicity of oils used in coenzyme Q10 self-emulsifying drug delivery systems (SEDDS). Int. J. Pharm. 2004; 273:63–73.
- Goddeeris C. et al. Light scattering measurements on microemulsions: estimation of droplet sizes. Int. J. Pharm. 2006; 312:187–195.
- Vyas S.P., Khar R.K. Submicron emulsion. In Targeted and Controlled Drug Delivery Novel Carriers Systems. CBS Publishers and Distributors, 2002; pp. 291–294.
- Fatouros DG et al. Morphological observations on a lipid-based drug delivery system during in vitro digestion. Eur J Pharm Sci 2007; 31:85–94.
- Yadav A. Preparation and Evaluation of Herbal Mouthwash containing Psidium guajava Leaf Extract. International Journal of Pharmaceutical Research and Development. 2020; 2(1): 24-26.
- Greiner RW, Evans DF. Spontaneous formation of a water-continuous emulsion from water-in-oil microemulsion. Langmuir 1990; 6: 1793–1796.
- Yadav A, Raut I, Vakhariya R. Formulation & Evaluation of Herbal Lipstick using Morus alba Linn. International Journal of Pharmacy and Pharmaceutical Science. 2020; 2(1): 04-05.
- Porter CJH, Trevaskis NL, Charman WN. (2007). Lipids and lipid based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 6:231–8.
- Patil S, Yadav A, Chopade A, Mohite S. Design, Development and Evaluation of Herbal Mouthwash for Antibacterial Potency against Oral Bacteria. Journal of University of Shanghai for Science and Technology. 2020; 22(11): 881-898.1137-1148.
- Bowtle W. (2007). Materials, process, and manufacturing considerations for lipid-based hard- capsule formats. In: Hauss DJ, ed. Oral lipid based formulations enhancing the bioavailability of poorly water soluble drugs. vol. 170. New York: Informa Healthcare, 79–106.
- Honmane P, Mali V, Mali A, Mahadik P, Yadav A, Mohite S. Formulation and Evaluation of Herbal Ointment Containing Eclipta Alba (L.) Journal of Seybold Report. 2020; 25(10): 569-577.
- Dhadde Gurunath S., Mali Hanmant S., Raut Indrayani D., Nitalikar Manoj M., An Overview on Multiple Emulsions, Asian Journal of Pharmacy and Technology, 2021; 11(2): 156-158.
- Dange V, Dinde S, Doiphode A, Dhavane S, Dudhal B, Shid S, Yadav A. Formulation and Evaluation of Herbal gel Containing Lantana Camara for Management of Acne Vulgaris. Journal of University of Shanghai for Science and Technology. 2020; 22(11): 799-809.
- Ku MS, Li W, Dulin W, et al. (2010). Performance qualification of a new hypromellose capsule: Part I. Comparative evaluation of physical, mechanical and process ability quality attributes of Vcaps Plus_, QualiV and gelatin capsules. Int J Pharm 2013; 386:30–41.
- Yadav A, Mohite S. Formulation and Evaluation of Antidandruff Shampoo. Research Journal of Topical and Cosmetic Sciences. 2020; 11(2): 55-58.
- Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery system; preparation techniques and dosage forms. Drug Discov Today. 2013; 13:606–12.
- Caliph SM, Charman WN, Porter CJH. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph cannulated and non-cannulated rats. J Pharm Sci. 2000. 89:1073–84.
- Trevaskis NL, Charman WN, Porter CJH. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008; 60:702–16.
- Dahan A, Hoffman A. Rationalizing the selection of oral lipid based drug delivery system by an in vitro lipolysis model for improved oral bioavailability of poorly water soluble drugs. J Control Release. 2008; 129:1–10.
- Wasylaschuk WR, Harmon PA, Wagner G, et al. Evaluation of hydroperoxides in common pharmaceutical excipients. J Pharm Sci. 2007. 96: 106–16.
- Sato K. Crystallization behavior of fats and lipids-a review. Chem Eng Sci. 2007; 56: 2255–65.
